Progressive Retinal Atrophy in Cavaliers:
A Slow Death of the Retina
-
 Symptoms
Symptoms - Diagnosis
- DNA Testing
- Treatment
- Breeders' Responsibilities
- What You Can Do
- Research News
- Related Links
- Veterinary Resources
Progressive retinal atrophy (PRA), also known as progressive retinal degeneration (PRD), is a disease which causes blindness and has "increased incidence" and is "presumed inherited" in the cavalier King Charles spaniel.* PRA is inherited by an autosomal recessive gene. It is a slow but progressive degeneration or death of the retinal tissue. It is similar to retinitis pigmentosa in humans.
All CKCSs should be examined at least annually by a board certified veterinary ophthalmologist. They are listed on the website of the American College of Veterinary Ophthalmologists.
* See the Canine Inherited Disorders Database and Ophthalmic Disease in Veterinary Medicine.
RETURN TO TOP
Symptoms
It's earliest signs may be overlooked, and owners may not notice the early stages of the disorder. It usually begins with an an abnormal shine in the cavalier's eyes. This is due to the pupils being dilated and not responding as quickly to light as pupils of normal dogs. Other early indications include night vision difficulties that usually progress to day blindness.
Late in the progression of PRA, cataracts may develop. See Cataracts for more information.
RETURN TO TOP
Diagnosis
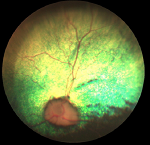 Diagnosis should be conducted by a veterinary ophthalmologist. Usually the
veterinarian first examines the dog with an indirect ophthalmoscope. An
electroretinogram (ERG) is essential for an accurate diagnosis, because
cataracts also may develop in the eyes of dogs suffering from PRA, and they may
interfere with the ophthalmologist's examination of the retina. Examination
using the ERG may require that the dog be anesthetized. The ERG is sensitive
enough to diagnose dogs with PRD before they begin to demonstrate signs of the
disease.
Diagnosis should be conducted by a veterinary ophthalmologist. Usually the
veterinarian first examines the dog with an indirect ophthalmoscope. An
electroretinogram (ERG) is essential for an accurate diagnosis, because
cataracts also may develop in the eyes of dogs suffering from PRA, and they may
interfere with the ophthalmologist's examination of the retina. Examination
using the ERG may require that the dog be anesthetized. The ERG is sensitive
enough to diagnose dogs with PRD before they begin to demonstrate signs of the
disease.
RETURN TO TOP
 DNA Testing
DNA Testing
Progressive rod-cone degeneration (PRCD) is an inherited form of
late-onset PRA, causing autosomal recessive retinal degeneration in
dogs. While no veterinary studies have discovered this form of PRA as
inherited in the CKCS, other spaniel breeds, particularly the American
and English cocker and English springer spaniels, as well as toy and
miniature poodles, have been found to have a mutation of the PRCD gene
in the
 region of canine chromosome 9 (CFA9). Mixed breed dogs which
include cavaliers with these breeds also may be particularly susceptible
to this mutation.
region of canine chromosome 9 (CFA9). Mixed breed dogs which
include cavaliers with these breeds also may be particularly susceptible
to this mutation.
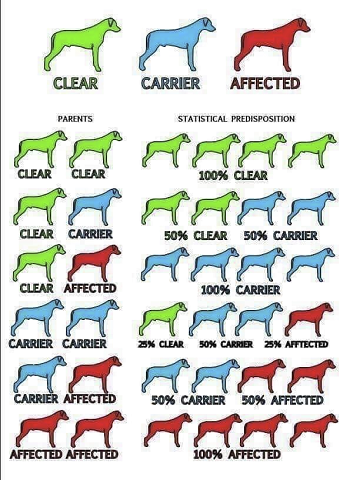
Dogs with a single mutation (genotype) are carriers but not affected. Dogs with two mutations are expected to develop this form of PRA and can transmit it to all of their offspring.
Genetic testing laboratories which test for this mutation causing PRCD include:
• Canine Genetic Testing (CAGT), University of Cambridge Dept. of Veterinary Medicine
• DDC DNA Diagnostics Center
• Dog Breeding Science (Australia)
• Embark, Cornell University College of Veterinary Medicine
• GenSol Animal Diagnostics
• Laboklin
• Paw Print Genetics
• Veterinary Genetics Laboratory
![]() Watch
this YouTube video to learn how to properly use a swab to obtain DNA from
your dog. See our Genetic Testing webpage for
more information about DNA testing.
Watch
this YouTube video to learn how to properly use a swab to obtain DNA from
your dog. See our Genetic Testing webpage for
more information about DNA testing.
RETURN TO TOP
Treatment
There currently is no medical treatment for PRA. However, research is being conducted. See the December 2014 item below under Research News.
RETURN TO TOP
Breeders' Responsibilities
 The Canine Inherited Disorders Database recommends that cavalier King Charles
spaniels suffering from PRA not be bred. Any littermates of breeding stock
having PRA should be taken into consideration.
The Canine Inherited Disorders Database recommends that cavalier King Charles
spaniels suffering from PRA not be bred. Any littermates of breeding stock
having PRA should be taken into consideration.
The Cavalier King Charles Spaniel Club, USA recommends that, prior to breeding any cavalier, the dog have a normal rating from a screening by a board certified veterinary ophthalmologist.
The Canine Health Information Center (CHIC) is a centralized canine health database sponsored by the AKC/Canine Health Foundation (AKC/CHF) and OFA. The CHIC, working with participating parent clubs, provides a resource for breeders and owners of purebred dogs to research and maintain information on the health issues prevalent in specific breeds.
AKC's national breed clubs establish the breed specific testing protocols. Dogs complying with the breed specific testing requirements are issued CHIC numbers. The ACKCSC requires that, to qualify for CHIC certification, cavaliers must have a CERF eye examination, recommending that an initial CERF exam be performed at 8 to 12 weeks, with a follow up exam once the dog reaches 12 months, and annual exams thereafter until age 5 years, and every other year until age 9 years. However, all that is required to qualify for a CHIC certificate is that the breeding stock be examined by a veterinary ophthalmologist. It does not require that the results of the examination show no eye disorders.
Nevertheless, all cavalier breeding stock should be examined by board certified veterinary ophthalmologists at least annually and cleared by the veterinary specialists for PRA, the closer the examination to the breeding the better.
RETURN TO TOP
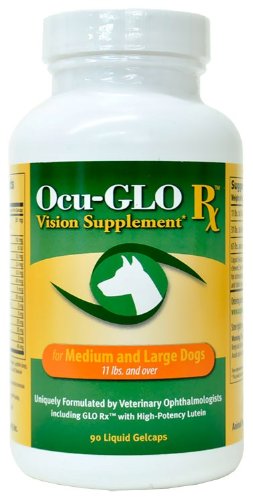 What You Can Do:
What You Can Do:
Ocu-GLO Rx is a nutraceutical containing several natural antioxidants in a combination blend formulated specifically for canine eye health. Many veterinary ophthalmologists recommend this product to maintain healthy eyes. Even if your dog has not been diagnosed with a vision disorder, antioxidants contained in Ocu-GLO Rx are considered helpful in keeping dogs' eyes healthy.
RETURN TO TOP
Research News
December 2014:
USA researchers find a new therapy which restores light sensitivity to
retina of dogs blinded by retinal atrophy.
 In a
December
2014 report, a team of University of Pennsylvania veterinary school,
PennVet, and University of
California - Berkeley researchers (Benjamin M. Gaub, Michael H. Berry,
Amy E. Holt, Andreas Reiner, Michael A. Kienzler, Natalia Dolgova,
Sergei Nikonov, Gustavo D. Aguirre, William A. Beltran, John G.
Flannery, Ehud Y. Isacoff) disclose that they have developed a new
genetic therapy which restored light response to the retinas of dogs
blind due to retinal atrophy.
In a
December
2014 report, a team of University of Pennsylvania veterinary school,
PennVet, and University of
California - Berkeley researchers (Benjamin M. Gaub, Michael H. Berry,
Amy E. Holt, Andreas Reiner, Michael A. Kienzler, Natalia Dolgova,
Sergei Nikonov, Gustavo D. Aguirre, William A. Beltran, John G.
Flannery, Ehud Y. Isacoff) disclose that they have developed a new
genetic therapy which restored light response to the retinas of dogs
blind due to retinal atrophy.
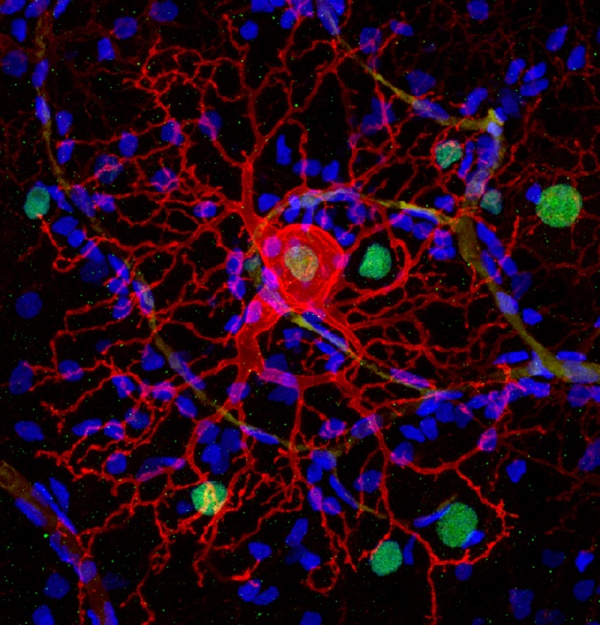
The therapy inserts a gene for a common ion channel into normally blind cells of the retina that survive after the light-responsive rod and cone photoreceptor cells die as a result of retinal atrophy diseases such as retinitis pigmentosa. Photoswitches in the form of chemicals that change shape when hit with light are then attached to the ion channels to make them open in response to light, activating the retinal cells and restoring light sensitivity. (Photo at right: A dog's retinal ganglion cell expressing LiGluR -- red -- can be controlled by light after delivering a chemical photoswitch.)
Lead researcher Ehud Isacoff stated:
“We’ve now showed that we can deliver the photoswitch and restore light response to the blind retina in the dog as well as in the mouse, and that the treatment has the same sensitivity and speed of response. We can reanimate the dog retina.”
More details about this research is available on-line here.
September 2014: Gene
defect responsible for PRA in Swedish valhund dogs is found.
 In
a September 2014 article, a team of researchers at the University of
Helsinki, Finland, and Michigan State University report they have
located a MERTK gene mutation believed to be responsible for a form of
progressive retinal atrophy in Swedish valhund dogs. Led by MSU Dr.
András M. Komáromy (right), the team examined 324 dogs in seven
countries and identified the defective gene that causes the disease. He
said that future work will include the search for the regulatory
mutation and study of overexpression-related disease mechanisms with a
possibility for a therapeutic option with MERTK inhibitors, and
developing a genetic marker test to reduce the frequency of PRA in the
Swedish vallhund breed.
In
a September 2014 article, a team of researchers at the University of
Helsinki, Finland, and Michigan State University report they have
located a MERTK gene mutation believed to be responsible for a form of
progressive retinal atrophy in Swedish valhund dogs. Led by MSU Dr.
András M. Komáromy (right), the team examined 324 dogs in seven
countries and identified the defective gene that causes the disease. He
said that future work will include the search for the regulatory
mutation and study of overexpression-related disease mechanisms with a
possibility for a therapeutic option with MERTK inhibitors, and
developing a genetic marker test to reduce the frequency of PRA in the
Swedish vallhund breed.
RETURN TO TOP
Related Links
RETURN TO TOP
Veterinary Resources
Control of Canine Genetic Diseases. Padgett, G.A., Howell Book House 1998, pp. 198-199, 241.
Breed Predispositions to Disease in Dogs & Cats. Alex Gough, Alison Thomas. 2004; Blackwell Publ. 44-45.
Ophthalmic Disease in Veterinary Medicine. Charles L. Martin. Manson Publ. 2009; page 475, table 15.1. Quote: "Presumed Inherited Ocular Diseases: Table 15.1: Breed predisposition to eye disease in dogs: Cavalier King Charles Spaniel: ... Progressive retinal atrophy (1-5 years of age)".
Progressive retinal atrophy, Canine Inherited Disorders Database. Quote: "What breeds are affected by progressive retinal atrophy? ... Cavalier King Charles spaniel."
Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Barbara Zanger, Orly Goldstein, Alisdair R. Philp, Sarah J.P. Lindauer, Susan E. Pearce-Kelling, Robert F. Mullins, Alexander S. Graphodatsky, Daniel Ripoll, Jeanette S. Felix, Edwin M. Stone, Gregory M. Acland, Gustavo D. Aguirre. Genomics. November 2006; doi: 10.1016/j.ygeno.2006.07.007. Quote: Progressive rod–cone degeneration (prcd) is a late-onset, autosomal recessive photoreceptor degeneration of dogs and a homolog for some forms of human retinitis pigmentosa (RP). Previously, the disease-relevant interval was reduced to a 106-kb region on CFA9, and a common phenotype-specific haplotype was identified in all affected dogs from several different breeds and breed varieties. Screening of a canine retinal EST library identified partial cDNAs for novel candidate genes in the disease-relevant interval. The complete cDNA of one of these, PRCD, was cloned in dog, human, and mouse. The gene codes for a 54-amino-acid (aa) protein in dog and human and a 53-aa protein in the mouse; the first 24 aa, coded for by exon 1, are highly conserved in 14 vertebrate species. A homozygous mutation (TGC → TAC) in the second codon shows complete concordance with the disorder in 18 different dog breeds/breed varieties tested. The same homozygous mutation was identified in a human patient from Bangladesh with autosomal recessive RP. Expression studies support the predominant expression of this gene in the retina, with equal expression in the retinal pigment epithelium, photoreceptor, and ganglion cell layers. This study provides strong evidence that a mutation in the novel gene PRCD is the cause of autosomal recessive retinal degeneration in both dogs and humans.
Guide to Congenital and Heritable Disorders in Dogs. Dodds WJ, Hall S, Inks K, A.V.A.R., May 2011, page 6 and Section II(256).
The genetics of eye disorders in the dog. Cathryn S. Mellersh. Canine Genetics & Epidemiology. April 2014. Quote: "Inherited forms of eye disease are arguably the best described and best characterized of all inherited diseases in the dog, at both the clinical and molecular level and at the time of writing 29 different mutations have been documented in the scientific literature that are associated with an inherited ocular disorder in the dog. The dog has already played an important role in the identification of genes that are important for ocular development and function as well as emerging therapies for inherited blindness in humans. Similarities in disease phenotype and eye structure and function between dog and man, together with the increasingly sophisticated genetic tools that are available for the dog, mean that the dog is likely to play an ever increasing role in both our understanding of the normal functioning of the eye and in our ability to treat inherited eye disorders. This review summarises the mutations that have been associated with inherited eye disorders in the dog."
A Novel Form of Progressive Retinal Atrophy in Swedish Vallhund Dogs. Ann E. Cooper, Saija Ahonen, Jessica S. Rowlan, Alison Duncan, Eija H. Seppälä, Päivi Vanhapelto, Hannes Lohi, András M. Komáromy. PLoS ONE. Sept. 2014;9(9):e106610. Quote: "The term progressive retinal atrophy (PRA) is used to describe inherited photoreceptor degeneration resulting in progressive vision loss. Because of the similarities in ocular anatomy, including the presence of a cone photoreceptor-rich central retinal region, and the close genotype-phenotype correlation, canine models contribute significantly to the understanding of retinal disease mechanisms and the development of new therapies. The screening of the pure-bred dog population for new forms of PRA represents an important strategy to establish new large animal models. By examining 324 dogs of the Swedish vallhund breed in seven countries and across three continents, we were able to describe a new and unique form of PRA characterized by the multifocal appearance of red and brown discoloration of the tapetal fundus followed over time by thinning of the retina. We propose three stages of the disease based on the appearance of the ocular fundus and associated visual deficits. Electroretinography revealed a gradual loss of both rod and cone photoreceptor-mediated function in Stages 2 and 3 of the disease. In the few dogs that suffered from pronounced vision loss, night-blindness occurred first in late Stage 2, followed by decreased day-vision in Stage 3. Histologic examinations confirmed the loss of photoreceptor cells at Stage 3, which was associated with the accumulation of autofluorescent material in the adjacent retinal pigment epithelium. Pedigree analysis was suggestive of an autosomal-recessive mode of inheritance. Mutations in six known canine retinal degeneration genes as well as hypovitaminosis E were excluded as causes of the disease. The observed variability in the age of disease onset and rate of progression suggest the presence of genetic and/or environmental disease modifiers."
Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Benjamin M. Gaub, Michael H. Berry, Amy E. Holt, Andreas Reiner, Michael A. Kienzler, Natalia Dolgova, Sergei Nikonov, Gustavo D. Aguirre, William A. Beltran, John G. Flannery, Ehud Y. Isacoff. Proceedings of the National Academy of Sciences. December 2014. Quote: "Most inherited forms of blindness are caused by mutations that lead to photoreceptor cell death but spare second- and third-order retinal neurons. Expression of the light-gated excitatory mammalian ion channel light-gated ionotropic glutamate receptor (LiGluR) in retinal ganglion cells (RGCs) of the retina degeneration (rd1) mouse model of blindness was previously shown to restore some visual functions when stimulated by UV light. Here, we report restored retinal function in visible light in rodent and canine models of blindness through the use of a second-generation photoswitch for LiGluR, maleimide-azobenzene-glutamate 0 with peak efficiency at 460 nm (MAG0460). In the blind rd1 mouse, multielectrode array recordings of retinal explants revealed robust and uniform light-evoked firing when LiGluR-MAG0460 was targeted to RGCs and robust but diverse activity patterns in RGCs when LiGluR-MAG0460 was targeted to ON-bipolar cells (ON-BCs). LiGluR-MAG0460 in either RGCs or ON-BCs of the rd1 mouse reinstated innate light-avoidance behavior and enabled mice to distinguish between different temporal patterns of light in an associative learning task. In the rod-cone dystrophy dog model of blindness, LiGluR-MAG0460 in RGCs restored robust light responses to retinal explants and intravitreal delivery of LiGluR and MAG0460 was well tolerated in vivo. The results in both large and small animal models of photoreceptor degeneration provide a path to clinical translation. Significance: We restored visual function to animal models of human blindness using a chemical compound that photosensitizes a mammalian ion channel. Virus-mediated expression of this light sensor in surviving retinal cells of blind mice restored light responses in vitro, reanimated innate light avoidance, and enabled learned visually guided behavior. The treatment also restored light responses to the retina of blind dogs. Patients that might benefit from this treatment would need to have intact ganglion cell and nerve fiber layers. In general, these are patients diagnosed with retinitis pigmentosa and some forms of Leber congenital amaurosis. Patients diagnosed with other types of blindness, for example, age-related macular degeneration or diabetic retinopathy, would not be candidates for this treatment."


CONNECT WITH US