Canine Chiari Malformation (CM) and Syringomyelia (SM) in the Cavalier King Charles Spaniel
-
 IN SHORT
IN SHORT - IN DEPTH
- Canine Chiari Malformation (CM)
- Syringomyelia
- Symptoms
- Diagnosis
- MRI Clinics
- DNA Testing
- Progression
- Treatment
- Breeders' Responsibilities
- What You Can Do
- Research News
- Related Links
- CM & SM In Other Breeds
- Veterinary Resources
- Page 2
- Page 3
IN SHORT:
Canine Chiari malformation (CM), also commonly known as Chiari-like malformation, is believed to play a major role in the cause of syringomyelia (SM) in cavalier King Charles spaniels. While some forms of SM are known to have other causes, this article focuses primarily upon its relationship with CM. CM/SM is multi-factorial, and therefore identifying no single gene mutation and avoiding it in later breedings will resolve this disorder in the breed.
CM is a complex skull and craniocervical junction malformation
associated with a short skull, that is common in some
brachycephalic toy
breed dogs and especially the cavalier (CKCS). The brain is
overcrowded in the skull, and there is also
overcrowding of the spinal cord in the upper neck vertebrae. In the
CKCS, this situation
 is compounded due to the cavalier having a
disproportionately large brain. The cavalier appears to have a brain more
appropriate for a bigger dog, about the size as that of a Labrador
retriever.l See
these YouTube videos by Dr. Rusbridge that fully explain what canine
Chiari malformation is in the cavalier.
is compounded due to the cavalier having a
disproportionately large brain. The cavalier appears to have a brain more
appropriate for a bigger dog, about the size as that of a Labrador
retriever.l See
these YouTube videos by Dr. Rusbridge that fully explain what canine
Chiari malformation is in the cavalier.
This disproportion causes the brain, particularly the cerebellum, to squeeze through the foramen magnum - the hole at the back of the skull, in the occipital bone - partially blocking the flow of cerebrospinal fluid (CSF) down the spinal cord. This both causes pain and the creation of fluid which collects in pockets in the spinal cord, which is what SM is. CM can cause irreversible damage to the spinal cord, resulting in additional pain and other neurological disorders.
SM is an extremely serious condition in which on or more of these "syrinxes" or "syringes", develop within the spinal cord near the brain. It is also known as "neck scratcher's disease", because one of its common signs is scratching in the air near the neck. "Syringomyelia" is Latin for "cavity within the spinal cord".
SM is rare in most breeds but has become very widespread in cavalier King Charles spaniels, the Brussels Griffon (Griffon Bruxellois), and Chihuahuas. The number of diagnosed cases in cavaliers has increased dramatically since 2000. Researchers estimate that more than 95% of cavaliers have CM and over 50% may have SM. The severity and extent of syringomyelia also appear to get worse in each succeeding generation of cavaliers. It is worldwide in scope and not limited to any country, breeding line, or kennel, and experts report that it is believed to be inherited in the cavalier. More ...
RETURN TO TOP
Symptoms
CM/SM seldom can be detected in young puppies, as symptoms of it usually are
not evident before the age of six months or years later.
Dogs diagnosed with CM and SM may have no outward symptoms at all. If CM-affected dogs do have symptoms, they indicate pain (CM-P). The most common ones are: (a) vocalization (barking, whining, moaning) particularly when being picked up under the chest or when changing position; (b) head scratching or head rubbing; (c) reduced activity, such as a reluctance to climb stairs or jump; (d) behavioral changes, such as becoming timid, anxious, or aggressive; and/or (e) touch aversion.
 SM-affected dogs may be asymptomatic if the syrinx is small and does not
interfere with the spinal cord. Larger syrinxes -- those having a
diameter of 4 mm or more -- can damage the spinal cord and cause
symptoms such as phantom scratching, scoliosis (see cavalier at
right), and weakness in the
limbs.
SM-affected dogs may be asymptomatic if the syrinx is small and does not
interfere with the spinal cord. Larger syrinxes -- those having a
diameter of 4 mm or more -- can damage the spinal cord and cause
symptoms such as phantom scratching, scoliosis (see cavalier at
right), and weakness in the
limbs.
Pain is the most important clinical sign of CM. Symptoms
may vary widely among different dogs, but the earliest sign often is that
the dog feels a hypersensitivity in its neck area, causing in some an uncontrollable
urge to scratch at its neck and shoulders. Then usually follows severe pain
around its head, neck, and
![]() shoulders, causing it yelp or scream.
Click here
or the YouTube logo to see videos of cavaliers with CM/SM symptoms. As the
disease progresses, it destroys portions of the cavalier's spinal cord, and
is so painful that the affected dog may contort its neck and even sleep and
eat only with its head held high. The dog's legs may become progressively
weaker, so that walking becomes increasingly difficult. Some dogs
deteriorate to the point of paralysis. More ...
shoulders, causing it yelp or scream.
Click here
or the YouTube logo to see videos of cavaliers with CM/SM symptoms. As the
disease progresses, it destroys portions of the cavalier's spinal cord, and
is so painful that the affected dog may contort its neck and even sleep and
eat only with its head held high. The dog's legs may become progressively
weaker, so that walking becomes increasingly difficult. Some dogs
deteriorate to the point of paralysis. More ...
RETURN TO TOP
Diagnosis
The only accurate way of confirming diagnosis of the disease is through the use of magnetic resonance imaging (MRI) scanning, which can be an extremely costly procedure. The MRI allows the veterinary neurologist to study the spine for the presence of any abnormality which might obstruct the flow of the cerebrospinal fluid. Accurate MRI results require that the dog be anesthetized. Clinic charges for MRI examinations of canines have been known to vary from a rare discounted rate of $600.00 to over $2,000.00.
The names and locations of veterinary neurologists who are board certified by the American College of Veterinary Internal Medicine (ACVIM) are on our Neurologists webpage. Chiari Check is an on-line questionnaire based diagnostic tool which gives a risk of Chiari-pain and syringomyelia.
Another disorder common to cavaliers and with symptoms similar to CM/SM is Primary Secretory Otitis Media (PSOM), which is a highly viscous mucus plug which fills the middle ear and causes the tympanic membrane to bulge. Because the pain and other sensations in the head and neck areas, resulting from PSOM, are so similar to symptoms due to SM, the possibility that the cavalier has PSOM and not SM should be determined before diagnosing SM. More ...
RETURN TO TOP
Treatment
Treatment options for CM/SM are very limited. But first of all, it is important to distinguish SM with symptoms from SM without symptoms. As a general rule, CM/SM without symptoms (asymptomatic) should not be treated with drugs.
Anticonvulsants, such as gabapentin
(Neurontin, Gabarone),
have been successful in some more severe cases.
 Pregabalin (Lyrica,
Accord, Alzain, Lecaent, Milpharm, Prekind, Rewisca, Sandoz, Zentiva), amitriptyline (Elavil, Tryptizol,
Laroxyl, Sarotex), and oral opioids (pethidine or methadone) are alternatives.
Methylsulfonylmethane (MSM) is recommended by some veterinary neurologists
as a dietary supplement.
Pregabalin (Lyrica,
Accord, Alzain, Lecaent, Milpharm, Prekind, Rewisca, Sandoz, Zentiva), amitriptyline (Elavil, Tryptizol,
Laroxyl, Sarotex), and oral opioids (pethidine or methadone) are alternatives.
Methylsulfonylmethane (MSM) is recommended by some veterinary neurologists
as a dietary supplement.
Drugs which reduce the production of cerebrospinal fluid, including proton pump inhibitors such as omeprazole (Prilosec), and the diuretic, furosemide (Lasix, Diuride, Frudix, Frusemide), and spironolactone (Aldactone), may be useful, but clinical data on their use and effectiveness is lacking. Carbonic anhydrase inhibitors, such as acetazolamide (Diamox) also serve to decrease the flow of cerebrospinal fluid, but their adverse side effects of abdominal pain, lethargy, and weakness limit long term use.
Before the disease progresses to its severe form, the use of cortisteroids, such as prednisolone, or non-steroidal anti-inflammatory drugs (NSAIDs, such as Rimadyl and Metacam) may relieve the symptoms but not the deterioration. Cortisteroids have serious side effects, such as weight, gait, and skin changes, and harmful suppression of the immune system. Long term use of these drugs is not advised. As a general rule, they should be reserved for a last resort, although some neurologists will start initial treatment of symptomatic dogs with a combination of an anticonvulsants, such as gabapentin, and a none-inflammatory dose of prednisolone.
Surgery to allow the cerebrospinal fluid to flow normally may be necessary to reduce the pain and deterioration. However, such surgeries are technically difficult and should be performed only by specialists. In some cases a shunt is installed. Although surgery often is successful, it is very expensive, and many dogs either have a recurrence of the disease or still show signs of pain and scratching. The most frequent reason for recurrence reportedly is the development of post-operative scar tissue. At least one neurologist has been inserting titanium mesh, in an effort to prevent such scar tissue from building up. More ...
RETURN TO TOP
Breeders' Responsibilities
CM/SM has a tendency to be more severe in each subsequent generation, and with an earlier onset. Breeders should follow the SM Breeding Protocol. The aim of the breeding protocol is to reduce the incidence of symptomatic syringomyelia in the cavalier breed, and not to create litters of puppies guaranteed not to have SM. The chance of producing an affected dog cannot be predicted without knowing the inheritance.
RETURN TO TOP
What You Can Do
• Send MRI scans of cavaliers 5 years old or older and which do not have SM, along with MRIs of those dogs' family members, to Dr. Clare Rusbridge at c.rusbridge@surrey.ac.uk.
• When lifting the dog, take care to support its entire body, including its head and neck. See this YouTube video as an example of how to lift the dog.
• Avoid "triggers" which can prompt a CM and/or SM reaction in the dog. The main trigger to avoid is walking the dog on a leash. Off leash exercise is preferable.
• Being professionally groomed can be extremely distressing for CM/SM-affected dogs, because the hands-on contact is constantly triggering.
 • Ease
your dog's symptoms by using a comfortable harness instead of a collar
attached to a
leash. The neck is one of the most vulnerable regions of the dog's
body. It houses the spinal cord, vertebrae, muscles, the
tongue bone, the thyroid gland, the trachea, the esosphagus, major
blood vessels, lymph nodes, and the thymus. Pulling on the collar
can permanently damage any or all of these vital features.
• Ease
your dog's symptoms by using a comfortable harness instead of a collar
attached to a
leash. The neck is one of the most vulnerable regions of the dog's
body. It houses the spinal cord, vertebrae, muscles, the
tongue bone, the thyroid gland, the trachea, the esosphagus, major
blood vessels, lymph nodes, and the thymus. Pulling on the collar
can permanently damage any or all of these vital features.
One of the best harnesses for cavaliers with CM/SM symptoms is the BRILLIANT K9 "Lucy Small" harness. It is easy to put on and easy to take off. Watch the videos: "Opening the harness" and "Walking the dog with the harness".
RETURN TO TOP
IN DEPTH:
- Canine Chiari Malformation (CM)
- Syringomyelia
- Symptoms
- Diagnosis
- MRI Clinics
- DNA Testing
- Progression
- Treatment
- Breeders' Responsibilities
- What You Can Do
- Research News
- Related Links
- CM & SM In Other Breeds
- Veterinary Resources
- Page 2
- Page 3
Canine Chiari malformation (CM)
- introduction to CM
- terminology of CM
- definitions of CM
- cause of CM
- recent CM research findings
- archive of prior CM research
- other factors leading to SM
- pain due to CM
For a thorough, current review of research into canine Chiari
malformation (CM) and its diagnosis and treatment, read
 Dr. Clare Rusbridge's
June 2020 article, "New considerations about Chiari-like malformation,
syringomyelia and their management", linked here.
Dr. Clare Rusbridge's
June 2020 article, "New considerations about Chiari-like malformation,
syringomyelia and their management", linked here.
See also, these YouTube videos by Dr. Rusbridge that fully explain what canine Chiari malformation is in the cavalier.
Introduction to CM
 Canine
Chiari malformation (CM) (Chiari-like malformation) is believed to play a major role in the cause of
syringomyelia (SM)
in cavalier King Charles spaniels. While some forms of SM are known to
have other causes, this article focuses primarily upon its relationship
with CM.
Canine
Chiari malformation (CM) (Chiari-like malformation) is believed to play a major role in the cause of
syringomyelia (SM)
in cavalier King Charles spaniels. While some forms of SM are known to
have other causes, this article focuses primarily upon its relationship
with CM.
Cerebrospinal fluid (CSF) surrounds the spinal cord and the brain. The brain, which is relatively quite heavy, literally is suspended -- floating -- within the CSF, which is contained in a three layered membrane -- a sack -- called the meninges. This serves to facilitate waste clearance, regulates intracranial pressure, and also insulates the brain from injury as it floats within it. CSF normally flows back and forth between the brain and spinal cord with each heart beat. Disruptions in the flow of CSF can lead to severe neurological conditions and play a role in both CM and SM in cavaliers.
For more detailed information about CSF, watch Dr. Clare Rusbridge's February 2025 YouTube video linked here.
CM is a complex skull and craniocervical junction malformation associated with a short (brachycephalic) skull, that is common in some brachycephalic toy breed dogs and especially the cavalier King Charles spaniel (CKCS). The skull is too small for the brain and there is also overcrowding of the spinal cord in the upper neck vertebrae. This is the result of premature closure of the growth plates -- the joints between the various bones of the skull. This is a congenital disorder called "complex craniosynostosis". In the CKCS, this situation is compounded due to the cavalier having a disproportionately large brain. The cavalier appears to have a brain more appropriate for a bigger dog, about the size as that of a Labrador retriever.
This disproportion causes the brain, particularly the cerebellum, to squeeze through the foramen magnum - the hole at the back of the skull, in the occipital bone - partially blocking the flow of cerebrospinal fluid (CSF) down the spinal cord. This both causes pain and the creation of fluid which collects in pockets in the spinal cord, which is what SM is. CM can cause irreversible damage to the spinal cord, resulting in additional pain and other neurological disorders.
CM is an inherited disorder which is rare in most breeds but reportedly has become very widespread in cavalier King Charles spaniels (CKCS) and the Brussels Griffon (Griffon Bruxellois) and Chihuahuas. Some researchers estimate that as many as 95% of CKCSs may have Chiari-like malformation (CM or CLM), the skull bone malformation believed to be a part of the cause of syringomyelia, and that more than 50% of cavaliers may have SM.* It is worldwide in scope and not limited to any country, breeding line, or kennel, and experts report that it is inherited in the cavalier King Charles spaniel. CM is so widespread in the cavalier that it may be an inherent part of the CKCS's breed standard.
*A 2011 study of 555 UK cavaliers, reported by their owners to be symptom-less, found 25% of one year olds and 70% of 6+ year olds had SM. However, in a 2015 study of the veterinary records of 3,860 CKCSs in the UK and Australia from 2009 to 2014, only 37 were diagnosed by MRI as being affected with CM/SM and an additional 84 cavaliers were suspected of being affected. In a June 2018 study of 339 symptom-less German cavaliers, MRI scans showed that 163 (48.1%) had SM.
CM may first appear at any age, although many dogs (up to 45%) will develop first signs of CM before their first birthday. As many as 15% will develop signs as of middle-age (between ages six and eight years.
CM can be progressive, in the sense that over a period of several months, the length of the cerebellar herniation can increase significantly. However, the severity of CM in a dog does not predict the presence of syringomyelia in that dog. Other factors may influence the development of a syrinx.
See Karen Kennedy's* Understanding Canine Chiari Malformation and Syrningomyelia for diagrams of the occipital bone and foramen magnum.
* Posted with the permission of Karen Kennedy, RTMR, MappSc, a magnetic resonance imaging specialist with The London Health Sciences Centre, London, Ontario, Canada. She prepared these diagrams on behalf of the Health & Education Committee of the CKCSC of Canada.
RETURN TO TOP
Terminology of CM
These four terms -- (1) Canine Chiari malformation, (2) Chiari-like malformation, (3) Caudal occipimalformation syndrome (COMS), and (4) Occipital hypoplasia (OH) -- have been used to identify the malformation believed to play a role in the cause of syringomyelia. Although they technically mean different things, they often are used interchangeably. Some neurologists prefer one term over the others. However, researchers meeting at the International Conference on Syringomyelia at the Royal Veterinary College in London in November 2006 agreed upon the use of Chiari-like malformation (CM or CLM) to describe the malformation found in the Cavalier and to a lesser extent in a few other brachycephalic breeds.
More recently, Canine Chiari malformation has been used to shorten and better describe the name of the disorder. Because prior to the November 2006 London conference, CM and OH and COMS all were used to describe the same malformation, they all are used interchangeably in this article.
• Caudal Occipital Malformation Syndrome (COMS)
The term, Caudal Occipital Malformation Syndrome (COMS) had been used, particularly by some specialists in the United States, to describe the disorder. Some diehard neurologists persist in using this term when referring to Chiari-like malformation in cavaliers. The authors of a 2012 German article insist that:
"... [T]he Chiari-like malformation in the Cavalier King Charles spaniel is characterized by indentation of the occipital (bone) with cerebellar herniation and is more correctly termed caudal occipital malformation syndrome."
• Occipital Hypoplasia (OH)
Occipital hypoplasia (OH) has been used to describe the displacement of the cerebellum into the area of the foramen magnum and a kinking of the medulla and an indentation of the cerebellum. "Hypoplasia" is a medical term defined as underdevelopment or incomplete development, and so, "occipital hypoplasia" in this instance means an underdeveloped or incompletely developed occipital bone, which is part of the back of the skull. However, at the November 2006 London conference, this term was rejected because there is no proof yet that the condition is related to a hypoplastic occipital bone. The actual disorder is believed to be caused either by an unusually small occipital bone or a confining membrane within the occipital bone, resulting in the cavity in the skull containing the cerebellum to be too small to fully contain it, leading to overcrowding of the caudal fossa and obstruction of the neural structures, including the incomplete closure or development of the neural tube through which flows the cerebrospinal fluid (CSF).
In a January 2009 article, researchers concluded that: "While several factors are associated with neurologic signs [of SM], occipital hypoplasia appears to be the most important factor."
However, in a June 2012 article, German researchers compared the volumes of occipital bones of cavaliers with and without syringomyelia and of French bulldogs. They did not find a reduced volume of the occipital bone of CKCSs, compared to the bulldogs. They concluded: "These results do not support occipital hypoplasia as a cause for syringomyelia development, challenging the paraxial mesoderm insufficiency theory."
Occipital hypoplasia is to be distinguished from occipital dysplasia, which is an incomplete ossification of the supraoccipital bone, causing a widening of the foramen magnum. The more brachycephalic is the shape of the dog's skull, the more likely there will be occipital dysplasia. The cavalier is a brachycephalic breed, and therefore a combination of both occipital hypoplasia and occipital dysplasia can occur in the CKCS. In a 2008 German study, the researchers recommend that cavaliers be screened for both occipital hypoplasia and occipital dysplasia.
In a December 2018 article, a team of Romanian and German researchers used computed tomography (CT) to diagnose CM, SM, and occiptial hypoplasia in a 21-month-old female cavalier King Charles spaniel. CT showed a typical brachycephalic head conformation, shortened facial bones, and a dome shaped calvarium. The supraoccipital bone was short and stunted, and the foramen magnum appeared enlarged, with part of the cerebellar vermis protruding. Evidence of a syrinx was observed in the spinal cord at C2. Changes of the occipital bone showed occipital hypoplasia with incomplete formation of the bone (See Fig. 6, below).
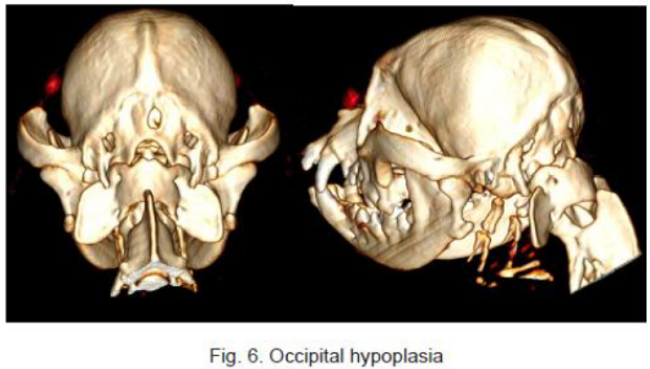
RETURN TO TOP
Definitions of CM
- Most Recent Definitions of Canine Chiari Malformation
- Previous Definitions of Canine Chiari Malformation
Canine Chiari malformation (CM) has had a variety of definitions over the years as more is learned about its likely causes.
• Most Recent Definitions of Canine Chiari Malformation
The most recent definition -- in this July 2018 article -- attempts to include in a general fashion all of the distortions found in the skulls of CM-affected dogs:
"CM might be described as any distortion of the skull and craniocervical junction which compromises the neural parenchyma and cerebrospinal fluid circulation causing pain and/or SM."
In this other July 2018 article, it was proposed that the disorder might be better described as a brachycephalic obstructive cerebrospinal (CSF) channel syndrome (BOCCS) with similarities to brachycephalic obstructive airway syndrome (BOAS).
Canine Chiari malformation is named after a similar condition in humans, discovered by Dr. Hans Chiari. Researchers estimate that up to 95% of CKCSs may have CM. The back half of the cavalier's skull typically may be too small to accommodate all of the brain's cerebellum, which may also be too large, and so it squeezes through the foramen magnum - the hole at the back of the skull, in the occipital bone - partially blocking the flow of cerebrospinal fluid (CSF) down the spinal cord. This is called cerebellar herniation. The variable pressure created by the abnormal flow of CSF is believed to create the SM cavities - called syrinxes - in the spinal cord. The cavalier appears to have a brain more appropriate for a bigger dog, about the size as that of a Labrador retriever.
• Previous Definitions of Canine Chiari malformation
Previously to 2010, CM was defined as "decreased caudal fossa volume with caudal descent of the cerebellum, and often the brainstem, into or though the foramen magnum." The 2010 definition was "a condition characterized by a mismatch in size between the brain (too big) and the skull (too small). There is not enough room for the brain and the back part (cerebellum and medulla) is pushed out the foramen magnum."
In an October 2014 article, UK researchers found three different definitions of CM:
1. Indentation: "Indentation of the caudal aspect of the cerebellum --- defined as a concave, rather than flattened or convex, caudal border of the cerebellum."
2. Impaction: "Impaction of the cerebellar vermis into the foramen magnum --- defined as deformation of the shape of caudo-ventral vermis into a point such that the angle between lines drawn along the caudal and ventral borders of the cerebellum meet at an acute, rather than an obtuse, angle. This definition was considered analogous to descent into the foramen magnum that has been used previously." (In photo of a CKCS at right, black arrow points to malformation of the caudal fossa of the occipital bone with visualization of the vermis.)
3. Herniation: "Herniation of the cerebellar vermis through the foramen magnum --- defined as extension of the cerebellar vermis caudal to a line drawn between the ventral aspect of the supraoccipital bone (opisthion) and the caudal border of the basioccipital bone (basion)."
They concluded that only the "herniation" definition distinguishes CM-dogs because "there is a high prevalence of cerebellar indentation and impaction in the normal canine population, suggesting they are unreliable as defining factors for CM."
However, even prior to that change, in a February 2014 article, a neurology team studying the Griffon Bruxellois (Brussels Griffons) recommended a redefinition of CM, explaining:
"This study supports the view that CM is a multifactorial condition that includes the shortening of the entire basicranium, loss of convexity of the supraoccipital bone, invagination of the cerebellum under the occipital lobes and possibly by increased proximity of the atlas to the occiput. As a compensatory change, there is increased height of the rostral cranial cavity and lengthening of the dorsal cranial vault. Overcrowding in the caudal cranial fossa and the craniocervical junction is a defining feature. The study provides the basis of a quantitative assessment of CM which might identify risk of syringomyelia and suggests that CM should be redefined so that account is taken of the overcrowding of the entire cranial fossa and craniocervical junction with reorganization of the brain."
In a March 2016 study of Griffons Bruxellois, the definition was tweaked again, and this time, much more complexly worded, as follows:
"a more global cranium and craniocervical junction abnormally characterized by insufficiency of the supra and basioccipital bones with compensatory rostral cranium doming, shortening of the skull base and increased proximity of the cervical vertebrae to the occiput resulting in overcrowding of the neural parenchyma in the caudal fossa."
RETURN TO TOP
Cause of CM
- Why does the cerebellum squeeze through that hole?
- Why is the skull too small for the brain?
- Why do the growth plates close prematurely?
- Why cavaliers?
Canine Chiari malformation can be very deceiving, because the obvious problem it causes is that the cerebellum squeezes through the foramen magnum, which is the small hole at the back of the skull in the occipital bone. As noted above, definitions of CM prior to 2010 were limited to describing that specific condition. But that is only a consequence of CM -- the end result of it -- and does not explain its cause.
Why does the cerebellum squeeze through that hole? Beginning in 2010, an answer has been that the skull is too small for the brain, causing overcrowding of the brain, with that hole being the path of least resistence to that overcrowding. But that, too, is only a consequence of CM and not the underlying cause.
Why is the skull too small for the brain? The current answer to that question is a developmental defect which has been labeled "complex craniosynostosis". Complex craniosynostosis is the premature closure of the growth plates which are the joints between the various skull bones. Growth plates in the skull are called cranial sutures, which are fibrous joints which remain flexible as the puppy's brain grows in size and then gradually fuse their adjoining skull bones together. When these growth plates close prematurely, the size of the skull does not accommodate the growing brain, and so the brain and skull become mismatched with the skull being too small for the brain. This overcrowding of the brain causes it to rotate to an unintended position within the skull, resulting in brachycephalia as well as CM. So, the definition of CM evolved in 2018 to incorporate these findings.
The next question is: Why do the growth plates close prematurely? It clearly is a congenital disorder, since it occurs at birth and during the developmental stage of the puppy's skull and brain. It is so common among cavalier King Charles spaniels that it must be genetic in the breed. Finding the answer to that question remains to be done, and perhaps once it is answered, the definition of CM will change again.
Finally, the question arises: Why cavaliers? Assuming for now that the cause in CKCSs is genetic, the breed-specific cause may be due to efforts by cavalier breeders to conform it a defined "breed standard", particularly one associated with the definition of the shape of the head. The direct ancestors of the CKCS have been around since as early as the 1500s. Paintings over the past 500 years have shown toy spaniels having a variety of head shapes, but mostly (with notable exceptions) with fairly pointed (snipy) muzzles until the mid-1800s and much more shortened ones beginning in the early 1900s. This early-1900 version has persisted since then and is the current head shape of the King Charles spaniel (English toy spaniel). Then beginning in 1926 an effort was made by some UK breeders to re-establish the somewhat longer muzzle of the mid-1800s. (See Origin of the Breed.)
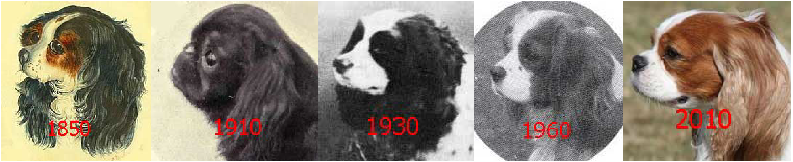
The current breed standard definition of the proper head shape of a cavalier is derived from the efforts of those late-1920s breeders to produce the longer muzzle. Specifically, the UK Cavalier Club's breed standard describes the ideal CKCS head shape as:
"Head and Skull: Skull almost flat between ears. Stop shallow. Length from base of stop to tip of nose about 3.8 cms (1½ ins). Nostrils black and well developed without flesh marks, muzzle well tapered. Lips well developed but not pendulous. Face well filled below eyes. Any tendency to snipiness undesirable."
Other nations' cavalier clubs' breed standards describing the proper head are very similar if not identical. So, over the past 500 years, the cavaliers' ancestors have gone from pointed (snipy) muzzles with fairly narrow heads to almost pug-shaped heads with exaggeragted domes of the early 1900s to the more moderate muzzles and wider skulls since 1926. These efforts have caused a sharp reduction in the gene pool of the breed, and particularly very likely several genes associated with the head, including the skull and the brain. Geneticists have found that when you encourage the mutation of one gene to achieve a certain specific result, you may end up with all sorts of other genetic consequences, called the "hitch-hiking effect". See this February 1974 article.
So, the cavalier breeders did not intentionally seek to create a breed with "a head too small for its brain", but with the hitch-hiking effect of mutated genes, that may be with what they ended up.
RETURN TO TOP
Recent CM research findings (2015 - 2025)
In a February 2021 article, neurology researchers Clare Rusbridge and Penny Knowler point out that there is increasing evidence that brachycephaly disrupts cerebrospinal fluid [CSF] movement and absorption, predisposing CM and SM. They show how the reduction of the lymphatic absorption of CSF through the lymphatic system organs located in the nasal and skull base, combined with the restriction of CSF movement through the junction of the skull with the spinal cord, appear to be key consequences of extreme brachycephaly in dogs and explain the likely causes o f these neurological disorders. Specifically relating to CM and SM, they state:
"Cavalier King Charles spaniels with syringomyelia associated with Chiari-like malformation have smaller volume jugular foramina compared to Cavalier King Charles spaniels without syringomyelia. However, direct causality between smaller jugular foramen and syringomyelia has not been proven. ... [C]raniosynostosis [premature closure of cranial sutures, preventing continued growth to accommodate the growing size of the brain] may be associated with a primary venous abnormality. Cavalier King Charles spaniels with syringomyelia associated with Chiari malformation have reduced volume caudal cranial fossa dorsal sinuses. ... Chiari-like malformation associated pain in dogs describes a syndrome of pain associated with brachycephaly and hindbrain herniation. It is often compared to Chiari type I and 0 malformation in humans. However, it is more like the hindbrain herniation seen with syndromic and complex craniosynostosis in humans, for example, Crouzon's and Pfeiffer syndrome."
"In comparison to dogs with Chiari-like malformation only, dogs with syringomyelia have more extreme brachycephaly with craniocervical junction deformation, including cervical flexure, change in angulation of the odontoid peg, increased proximity of the atlas to the skull (often referred to as atlanto-occipital overlapping), kinking or elevation of the craniospinal junction, and loss of the cisterna magna. Changes in conformation of the spinal canal and cord may also contribute. The authors propose that syringomyelia develops due to a combination of reduced CSF absorption though nasal lymphatics, reduced venous drainage, altered neuroparenchymal compliance, and reduced CSF movement through the lateral apertures or craniocervical junction. Curvature of the spinal canal and intrathoracic pressure gradient may contribute especially in the thoracic spinal cord. The mechanism of development of syringomyelia is controversial. The most accepted theory is that subarachnoid space obstruction results in a mismatch in timing between the arterial pulse peak pressure and CSF pulse peak pressure."
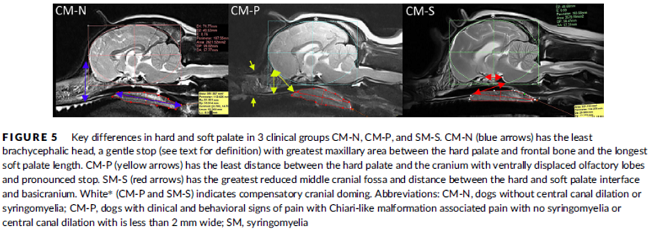
In a November 2019 article, a team of UK researchers reviewed the medical records of 66 cavaliers, 40 of which had syringomyelia (SM) and the other 26 did not; 55 had Chiari-like malformation (CM) and 11 did not. The dogs were grouped by (1) control group of 11 with no Chiari-like malformation (CM-N); (2) CM pain group (CM-P) of 15 dogs; (3) clinical SM group (SM-S) of 40 dogs. SM-S dogs included those with outward symptoms of SM (variable phantom scratching, scoliosis, etc.) and a syrinx of at least 4 mm. The researchers divided their study into two sub-sets, the first examined head features related to the dogs' soft palates, and the other examined features related to their hard palates; both sub-sets also included review of the dogs' features related to forebrain flattening and olfactory bulb rotation. The olfactory bulb is a bulb of neural tissue within the dog's fore-brain. Their work included comparing the shape of the "stop" of each dog, which is the degree of the angle where the nose and skull meet, and the indentation between the eyes at that point. A "gentle stop" has the least angular shape and a "pronounced stop" has the sharpest angle.
They found (see figure 5 below):
• CM-N dogs (no CM) had the least brachycephalic head, a gentle stop with the greatest upper jaw area between the hard palate and the frontal bone, and the longest soft palate length.
• CM-P dogs (painful CM) had the least distance between the hard palate and cranium, a pronounced stop, and a displaced olfactory bulb.
• CM-S dogs (large syrinx) had the most reduced middle craial bone area and shortest distance between the connection of the hard and soft palates with the base of the cranium.
They conclude that dogs with CM-P had the shortest muzzle lengths, and that "a reduced distance between the hard palate and the frontal bone was particularly associated with CM-P." Dr. Clare Rusbridge, one of the researchers, explained:
"Dogs with clinically relevant CM/SM are more likely to have brachycephalic features of the rostral skull flattening with reduction of nasal tissue and a well-defined stop. This evidence not only enhances our understanding of the disease and 'at risk' head conformation but could also impact on the assessment of MRI and disease diagnosis. It suggests the whole skull should be analyzed and not just the hindbrain currently required in prebreeding screening. This information has implications not only for breeders and pet owners but also for the veterinary profession to raise awareness about the welfare aspects of breeding. Furthermore, an increased risk for SM and painful CM might not be confined to brachycephalic breeds but other miniaturized purebreeds and hybrids that have gained in popularity as pets."
Co-researcher Dr. Susan P. Knowler explained:
"This study suggests that the whole skull, rather than just the hindbrain, should be analysed in diagnostic tests. It also impacts on how we should interpret MRI from affected dogs and the choices we make when we breed predisposed dogs and develop breeding recommendations. ... The brachycephalic features that can be seen from outside is a head that has flattening at the front with reduction of nasal tissue and a well-defined stop."
In a September 2019 article, a team of UK neurology researchers used "machine-learning" to identify biomarkers which distinguish between cavaliers with and without pain due to Chiari-like malformation (CM-P) and also those with and without syringomyelia (SM). Thirty-two CKCSs were included in the study, of which 10 had pain due to CM, 11 had symptomatic SM (SM-S), and 11 controls which had neither CM-P nor SM. "Machine-learning" is a process of a computer not explicitly programmed by people, which looks for patterns and data, then analyzes that information and draws conclusions and makes predictions from that gathered information. In this case, the machine looked for morphological changes in the dogs, which may not be apparent to human observers, thereby removing potential bias or blindness that may be produced by a hypothesis driven expert observer approach. The machine learning approach was to understand neuromorphological change and to identify image-based biomarkers in dogs with CM-P and and symptomatic SM (SM-S). Upon comparing dogs with CM-P or SM-S to the control group, candidate biomarkers were identified in specific regions of the brain for CM-P and for SM-S, particularly between the presphenoid bone and area between the soft palate and the tongue, which they concluded indicates both conditions being strongly related to changes within that area. This is a very preliminary study aimed at developing these biomarkers into a clinical diagnostic test.
In an April 2019 abstract, a team of UK neurology researchers (Eleonore Dumas [right], Susan Penny Knowler, Felicity Stringer, Clare Rusbridge) examined MRI scans of 66 cavalier King Charles spaniels, including 11 without either syringomyelia (SM) or painful Chiari-like malformation (CM-P), 15 with only CM-P, and 40 with clinically severe CM/SM. They report finding that the SM-affected CKCSs "had a more ventral orientation of the olfactory bulbs and shorter distance between basicranium and hard palate." They concluded that cavaliers with symptomatic CM/SM are more likely to have brachycephalic snouts and "'midface' hypoplasia similar to craniosyostosis Crouzan syndrome."
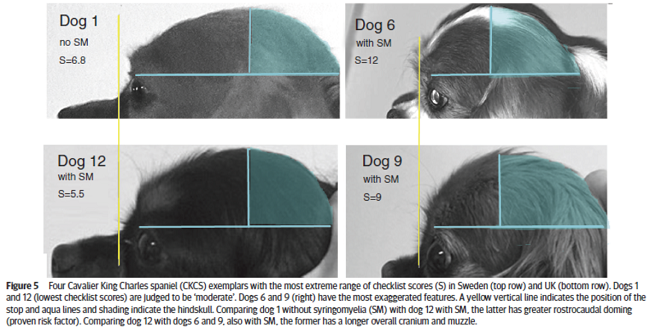
In a January 2019 article by a team of UK and Swedish researchers (Susan Penelope Knowler, Lena Gillstedt, Thomas J Mitchell, Jelena Jovanovik, Holger Andreas Volk, Clare Rusbridge), 13 cavalier King Charles spaniels were examined by UK breed judges, using a checklist, to determine if the risk of Chiari-like malformation (CM) and syringomyelia (SM) could be identified by visual assessment of head shape. The results showed a positive correlation between the judges' evaluations and the risk of CM/SM sufficient to warrant a larger study of the breed. Figure 5 (below) shows the most extreme range of checklist scores. The researchers concluded:
"This prospective investigation demonstrated that it was possible to compare subjective evaluation of head conformation with objective measurements and revealed a significant correlation between the subjective visual evaluation of head conformation and an objective evaluation of dorsoventral doming using photographs. However, this pilot investigation demonstrated that individual adjudicators can vary in their interpretation of the CKCS breed type and also suggests that measuring the cephalic index or rostrocaudal doming alone is not a reliable indicator of brachycephaly but should be taken together with a visual evaluation and take account of other features, such as those on the checklist and the size of the dog."
In a July 2018 article, Dr. Susan (Penny) Knowler (and Gabriel L. Galea, Clare Rusbridge) reviews over 20 years of neurological research into the conditions of Chiari-like malformation (CM) and syringomyelia (SM) primarily in cavalier King Charles spaniels (CKSC) and a few other brachcyphalic breeds. She covers the key morphocenetic processes involved in CM/SM, including (a) anatomical abnormalities, (b) brachycephaly, (c)cranio-cervical junction abnormalities, (e) embryology (fetal development), (f) the brain and ventricles, (g) the skull, and (h) genetics of CM. She provides a current defintion of CM as:
"a malformation of the skull and craniocervical junction which compromises the neural parenchyma to cause pain and/or disrupt CSF circulation which can result in SM."
In a January 2017 article, the UK researchers further pursued their analysis of CM resulting from an overall disorder of the conformation of the CKCS brain and skull. They stated:
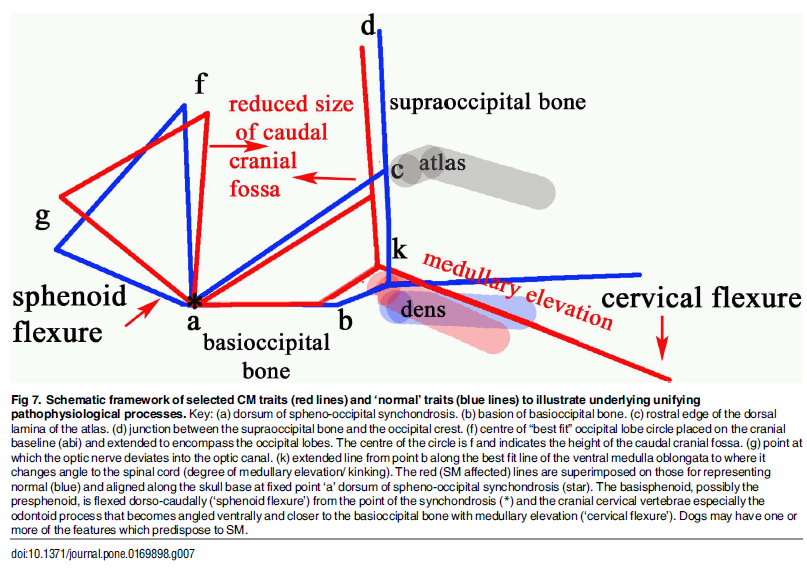
"Thus, CM is not just a reduction in the cranial base and caudal fossa. The `ellipticity' of the brain provides a quantitative value to compare the natural oval shape of the Control cohort to the more global brachycephalic CM pain and two SM cases. The reduced size and rotation of the olfactory bulb, together with the clival angle (cranial base angulation between the ethmoidal plane and the clival plane), is associated with a shortened muzzle and increased stop and a `face' that tilts up like a human. ... The morphing movie (S1 Movie) highlights the dynamic changes of the skull conformation and brain parenchyma associated with progressive brachycephaly and airorhynchy, shortening of the basicranium and supraoccipital bones and the proximity and angulation of the atlas and dens."
Describing cavaliers specifically, the researchers stated:
"Ten of the fourteen significant variables were found in the CKCS with one, line a-c [see red line a-c in the diagram below], unique to the breed. Line a-c indicates the proximity of the sphenooccipital synchondrosis to the atlas bone. This study confirms the findings of others that the CKCS with SM have a reduced caudal fossa size a presumed consequence of early closure of the spheno-occipital and possibly other cranial sutures. Compared to other breeds including the GB, the CKCS has considerably greater incidence of cerebellar deformation by the supra-occipital bone and vermis herniation. These findings and the coexistence of occipital dysplasia and hypoplasia suggest that the CKCS may have additional predisposing risk factors for SM compared to the other breeds."
In an April 2016 abstract, UK neurological researchers found evidence that CM may be the result of an overall disorder of the conformation of the CKCS brain and skull. They examined MRI scans of the skulls of 70 cavaliers, divided into four categories: SM with phantom scratching (15 CKCSs); clinical SM (e.g. pain) but no phantom scratching (17 CKCSs); behavioral signs of pain with CM but no SM (25 CKCSs); and CKCS with no SM and no behavioral signs of pain or scratching (13 dogs -- "CKCS control"). They also had an "other-breed-control" group of 19 dogs (including 5 brachycephalic -- short-muzzled), with normal brain sizes.
They
hypothesized that there may be insufficient room within the skull for
the forebrain, and that may contribute to backward displacement and
overcrowding of the hindbrain. They focused upon the forebrain's
olfactory bulb (OB -- also called olfactory lobe), which is at the lower front of the forebrain and
directly behind the olfactory receptor cells in the dog's nose. The more brachycephalic (short-muzzled) the dog, the
more the OB tends to be lower and the more the frontal lobe tends to be
flattened against the front of the skull. (Compare the normal
location of the canine forebrain in the diagram at the left, with the
flattened frontal lobe and the lower olfactory lobe of a CM/SM-affected
cavalier, at the right.)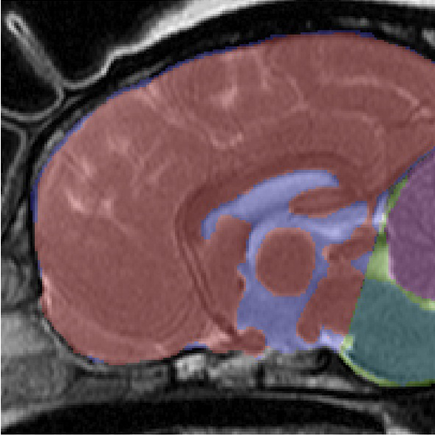
They found that the more severe the CM/SM condition of the cavaliers in the study, the smaller the mean size of the OB, and that there was a significant difference between the cavaliers in the four CM/SM groups and the dogs in the other-breed-control group. They also noticed a trend towards more ventrally (lower) orientated OB with increasing CM/SM severity. They concluded:
"This study suggests that CM should be considered a more global brain and skull conformational disorder with features of extreme brachycephaly including smaller more ventrally orientated OB; however, further work is required and the measurement technique has been refined for future studies. We recommend that future studies into MRI conformation of CM and SM uses rigorous phenotyping based on clinical signs and age."
In an
April 2015 article on the subject of the kinking (or elevation) of the medulla,
researchers examined 36 cavaliers (33 having canine Chiari malformation and 26
having
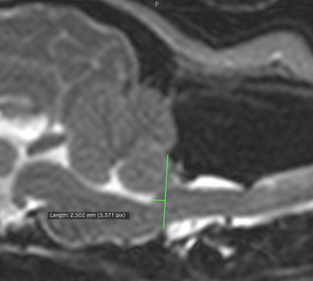 syringomyelia)
and reported finding that higher elevation of kinking of the medulla
related to neurological clinical signs of CM/SM. They also found that
brainstem position measurements at the caudodorsal-most border of the
fourth ventricle (called the "obex position") were associated with both
the presence and severity of syringomyelia. An obex position measurement
of ≤3.5 was sensitive (79%) and highly specific (90%) for the presence
of syringomyelia.
syringomyelia)
and reported finding that higher elevation of kinking of the medulla
related to neurological clinical signs of CM/SM. They also found that
brainstem position measurements at the caudodorsal-most border of the
fourth ventricle (called the "obex position") were associated with both
the presence and severity of syringomyelia. An obex position measurement
of ≤3.5 was sensitive (79%) and highly specific (90%) for the presence
of syringomyelia.
(The photo at right from the April 2015 article shows how the position of the brainstem was evaluated by measuring the distance between the obex (the caudodorsal-most border of the fourth ventricle) and a line drawn parallel to the foramen magnum. This was termed the "obex measurement". )
RETURN TO TOP
Archive of prior CM research
 There is not yet a consensus among veterinary investigators as to how to
measure the cavalier's occipital bone to determine what should be the shape
of the cerebellum within a "normal" CKCS's occipital bone.
Dr.
Clare Rusbridge, BVMS, MRCVS, PhD, DipECVN (right), of the Stone
Lion Veterinary Centre in London, England, a leading investigator into SM,
has described the three "classic features" of canine Chiari
malformation as:
(1) loss of the normal round shape of the cerebellum, which can appear to be
indented by the occipital bone; (2) displacement of the cerebellum into and
through the foramen magnum, i.e. herniation; and (3) kinking of the medulla.
2009 and 2010 UK studies in which Dr. Rusbridge later participated
(discussed below) suggest that caudal fossa volume may also play a role in
CM.
There is not yet a consensus among veterinary investigators as to how to
measure the cavalier's occipital bone to determine what should be the shape
of the cerebellum within a "normal" CKCS's occipital bone.
Dr.
Clare Rusbridge, BVMS, MRCVS, PhD, DipECVN (right), of the Stone
Lion Veterinary Centre in London, England, a leading investigator into SM,
has described the three "classic features" of canine Chiari
malformation as:
(1) loss of the normal round shape of the cerebellum, which can appear to be
indented by the occipital bone; (2) displacement of the cerebellum into and
through the foramen magnum, i.e. herniation; and (3) kinking of the medulla.
2009 and 2010 UK studies in which Dr. Rusbridge later participated
(discussed below) suggest that caudal fossa volume may also play a role in
CM.
In a 2006 study conducted by Dr. Natasha J. Olby and Dr. Sofia Cerda-Gonzalez, both board certified veterinary neurologists, and others at North Carolina State University's College of Veterinary Medicine's Department of Clinical Sciences and the IAMS Pet Imaging Center in Raleigh, NC., they have concluded that the incidence of caudal fossa and cervical spinal abnormalities is high in Cavaliers, and that the pathogenesis of syringomyelia is multi-factorial rather than due to a single malformation.
In a 2009 Scottish study led by Dr. Jacques Penderis, of 70 cavaliers and 80 dogs of other breeds, the researchers found that "all [of the] CKCSs had abnormalities in occipital bone shape. ... CKCSs had a shallower caudal cranial fossa and abnormalities of the occipital bone, compared with those of mesaticephalic dogs. These changes were more severe in CKCSs with syringomyelia."
However, in a January 2009 article, Drs. Sofia Cerda-Gonzalez, Natasha J. Olby, Susan McCullough, Anthony P. Pease, Richard Broadstone, and Jason A. Osborne failed to find the same association when comparing the caudal fossa of CKCS with and without syringomyelia using three-dimensional measurement methods.
Research journal articles published in 2009 and 2010 point to evidence that cavaliers' hind-skull volumes are not different from other small breeds, particularly those with short muzzles, and that the percentage of the volume of the caudal fossa -- the hind-skull cavity -- to the volume of the total cranial cavity, did not differ significantly between those CKCSs with and without SM.
However, these studies also found that the volume of hindbrain within the hind-skull was significantly greater for young -- 2-years and younger -- cavaliers with SM than older dogs -- 5 years and older -- without SM. They also found that increased hindbrain volume in CKCSs with SM, compared to that of the hind-skull, was directly correlated with the size of the dogs' syrinxes.
The first of these investigations was a 2009 German study of 40 cavaliers and 25 dogs of other brachycephalic breeds. The researchers found that: (1) "All CKCSs had cranial characteristics consistent with CLM"; and (2) "There were no significant differences between CKCSs and brachycephalic dogs with respect to the ... volumes of the CF [caudal fossa*] ...". They concluded: "Results of this study suggested that descent of the cerebellum into the foramen magnum and the presence of syringohydromyelia in CKCSs are not necessarily associated with a volume reduction in the CF of the skull."
* The caudal ( for "rear") cranial fossa is part of the cavity within the skull. It contains the brainstem and cerebellum, and towards its rear, it is enclosed by the occipital bone, which also frames the opening called the foramen magnum.
Similarly, in a 2009 UK study comparing the cerebral cranium volumes of the CKCS with those of other small breeds and the Labrador retriever, Hannah Cross and Drs. Rusbridge and Rodolfo Cappello found that cavaliers do not have a proportionately smaller caudal fossa compared to other small breeds, but that the CKCS's brain is comparatively large.
In that 2009 UK study, the researchers stated:
"When compared with Labradors, CKCS had proportionately the same volume of parenchyma [hindbrain] in their caudal fossa [skull], hence there is a mismatch of volumes with too much parenchyma in a too small caudal fossa causing overcrowding. ... Other small breeds of dogs had a proportionately smaller volume of parenchyma in their caudal fossa which can explain why, despite having a similar sized caudal fossa to CKCS, they do not experience overcrowding. It is hypothesised that through the miniaturisation process of other small dogs, both the cranium and brain are proportionately smaller but in CKCS only the cranium has reduced in volume, hence why there is a higher incidence of CM in CKCS than other small breeds.
"Cavalier King Charles spaniels also had a greater percentage of their cranial fossa filled with parenchyma (cranial fossa parenchyma percentage) compared with small breeds and Labradors which had a similar percentage. Overcrowding in CKCS might therefore occur due to a mismatch in volumes in both the caudal fossa and cranial fossa of the skull, suggesting the cranial fossa is also involved in the pathophysiology of CM."
They conclude:
"The results support mesoderm* insufficiency or craniosynostosis**as the pathogenesis of Chiari-like malformation (CM) in CKCS. It presents evidence for overcrowding of the caudal fossa due to a mismatch of brain parenchyma and fossa volumes as to why CKCS and not other small dogs are affected."
*The mesoderm is the middle of the three primary germ cell layers -- the others being ectoderm and endoderm -- in the early stage of an embryo. The mesoderm is responsible for developing various tissues and structures, such as bone, muscle, connective tissue, and the middle layer of the skin. Mesoderm insufficiency during embryology may cause insufficient scope for the mesoderm and ectoderm layers to develop.
**Craniosynostosis is a developmental abnormality in which the immature skull's growth plates prematurely fuse, changing the growth pattern of the skull.
This suggests both a possible genetic cause of the displacement of the cerebellum through the foramen magnum, as well as evidence that the cavalier's skull may not be too small, but that its hindbrain is too large, hence the "mismatch".
To the contrary, however, in a 2009 Scottish study led by Dr. Jacques Penderis, of 70 cavaliers and 80 dogs of other breeds, the researchers found that "all [of the] CKCSs had abnormalities in occipital bone shape. ... CKCSs had a shallower caudal cranial fossa and abnormalities of the occipital bone, compared with those of mesaticephalic dogs. These changes were more severe in CKCSs with syringomyelia."
 In a
2010 UK study report
in the Journal of Small Animal Practice (JSAP),
Colin J. Driver
(right), Dr. Clare Rusbridge, et al.
reiterated findings that
the variations in the dimensions of the cavaliers' posterior
[caudal] cranial
fossa* may not be associated with syringomyelia, since cavaliers do not have
a proportionately smaller caudal cranial fossa compared to other small
breeds. See, also, an abstract of that study
presented before the European College of Veterinary Neurology (ECVN).
In a
2010 UK study report
in the Journal of Small Animal Practice (JSAP),
Colin J. Driver
(right), Dr. Clare Rusbridge, et al.
reiterated findings that
the variations in the dimensions of the cavaliers' posterior
[caudal] cranial
fossa* may not be associated with syringomyelia, since cavaliers do not have
a proportionately smaller caudal cranial fossa compared to other small
breeds. See, also, an abstract of that study
presented before the European College of Veterinary Neurology (ECVN).
*The posterior (or caudal -- for "rear") cranial fossa is part of the cavity within the skull. It contains the brainstem and cerebellum.
The JSAP 2010 study researchers found that a cavalier with a higher volume of hindbrain within the skull is more likely to have SM, and the greater the volume of hindbrain, the larger the syrinx. They also found a direct relationship between between the dimensions of the brain ventricles ("ventriculomegaly" -- see below) and the size of the syrinx.
In addition, the 2010 JSAP research suggested that there may be a "failure of communication" between the paraxial mesoderm* and the cranial somites** with the closing neural tube*** in the embryo, resulting in loss of coordination between the growth of the skull and the hindbrain. When functioning properly, the growth of the mesoderm supports and helps to facilitate the closure process of the neural tube. They concluded that overgrowth of the cerebellum in the embryo may cause the mis-match, because cavaliers have proportionately more hindbrain volume than other small breed dogs. They stated: "Early growth plate closure may result in CM because despite the dynamic nature of osseous tissue, it would be unable to accommodate the developing brain."
*Paraxial mesoderm forms the supraoccipital bone.
**Cranial somitic mesoderm forms the exoccipital and basioccipital bones.
***The neural tube in the embryo develops the brain and spinal cord.
Then, later in 2010, the authors of the 2010 UK JSAP report presented an abstract before the 2010 congress of the British Small Animal Veterinary Association (BSAVA), in which they re-affirmed that, while SM occurs in cavaliers which have CM, it is the mis-match between the volumes of the hindbrain and the hind-skull which is believed to actually lead to SM, if not be the cause of SM. In that abstract, the authors go on to conclude that the more marked volume mis-matches they found between the hindbrain and the skull, the more severe the SM which affected the young dogs -- under 2 years of age -- in the study.
In a December 2010 UK study, led by Colin Driver, the researchers' results were consistent with the previous findings that ventriculomegaly and a small but significant increase in caudal fossa parenchyma are associated with syringomyelia. Further, this December 2010 study also found that the volume of the skulls of CKCS under 2 years of age and SM-affected were significantly smaller than the skull volumes of cavaliers over 5 years of age and SM-clear.
The UK studies in 2009 and 2010 suggest that a disproportionately large hind portion of the brain may be a necessary element of SM in the breed. These 2009 and 2010 research reports explain why CM has been re-defined as "a condition characterized by a mismatch in size between the brain (too big) and the skull (too small). There is not enough room for the brain and the back part (cerebellum and medulla) is pushed out the foramen magnum."
In a June 2011 study, which included Drs. Rusbridge, Driver, and McGonnell, they reported that twelve CM-affected cavaliers' foramen magnums and the length of cerebellar herniation "increased significantly" between MRI scans 9.5 months apart. they concluded:
"This work could suggest that overcrowding of the caudal cranial fossa in conjunction with the movements of cerebrospinal fluid and cerebellar tissue secondary to pulse pressures created during the cardiac cycle causes pressures on the occipital bone. This leads to a resorption of the bone and therefore an increase in caudal cranial fossa and foramen magnum size allowing cerebellar herniation length to increase."
 In an
April 2012 study by
Thomas A. Shaw, Imelda M. McGonnell, Colin
J. Driver, Clare Rusbridge, and Holger A. Volk, they
concluded that:
In an
April 2012 study by
Thomas A. Shaw, Imelda M. McGonnell, Colin
J. Driver, Clare Rusbridge, and Holger A. Volk, they
concluded that:
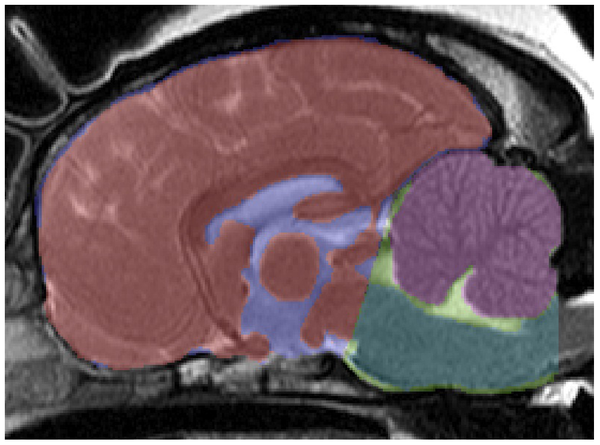
"the CKCS has a relatively larger cerebellum [in purple at right] than small breed dogs and Labradors and there is an association between increased cerebellar volume and SM in CKCS. In contrast to small breed dogs and Labradors, CKCS exhibit correlation between increased cerebellar volume and cerebellar crowding within the caudal CCF, suggesting that CCF growth in CKCS is not keeping pace with the growth of the cerebellum.
"These findings support the hypothesis that it is a multifactorial disease process governed by increased cerebellar volume and failure of the CCF to reach a commensurate size."
They also found:
(a) "CKCS under the age of 2 with SM have an increased cerebellar volume when compared to CKCS over the age of 5 without SM. This supports hypothesis that increased cerebellar volume in CKCS is associated with syringomyelia. Previous volumetric studies in CKCS have shown that there is an association between SM and CCF parenchyma volume, but this is the first time that cerebellar volume has been linked to SM. The cerebellum to brain volume ratio is consistent between normal dogs and has been shown to decrease with cerebellar degenerative disorders, but it has never been shown to be increased in size in a canine neurological disorder."
(b) "The degree of cerebellar crowding in the caudal CCF is correlated with increased volume of the cerebellum in CKCS, and this is not seen in small breed dogs or Labradors."
(c) "The degree of crowding may determine the degree of foramen magnum obstruction, and in turn the tendency for syrinxes to form. Cerebellar volume is potentially a key factor in determining the degree of obstruction and interference in normal CSF flow through the foramen magnum, which disposes dogs to the subsequent development of SM."
(d) "In CKCS an increase in relative cerebellar volume is correlated with an increase in cerebellar crowding in the caudal CCF. It should be noted that small breed dogs and Labradors do not show the same relationship. We infer from this result that during cranial development in Labradors and small breed dogs, a compensatory mechanism maintains the relationship between cerebellar volume and CCF dimensions, and this mechanism is defective in CKCS."
(e) "We also found in CKCS that cerebellar crowding in the caudal CCF is more sensitive to changes in relative cerebellar volume than cerebellar crowding in the rostral CCF, which is consistent with the theory that increased cerebellar volume results in the cerebellum shifting caudally and causes obliteration of dead space in the caudal CCF. This also causes herniation of the cerebellum through the foramen magnum (i.e. CM)."
(f) "In this study, we find that in CKCS, unlike small breed dogs or Labradors, there is a positive correlation between the volume of the cerebellum and degree of crowding in the caudal CCF, which suggests that CM may be due to CCF development not keeping pace with growth of the cerebellum. This supports the idea that CM/SM in CKCS may in fact be multifactorial and an abnormal development process affecting the CCF may be acting as a disease modifier."
(g) "Impaired CCF development may be caused by a failure of communication between one or more of these progenitors and the developing neural tube (specifically, rhombomere 1, which gives rise to the cerebellum). Alternatively, it could simply be explained by premature closure of growth plates between the bones of the CCF."
(h) "It has also been noted on post-mortem examination of CKCS and other small breed dogs that the supraoccipital bone overlying the cerebellar vermis is remarkably thin and sometimes eroded so that the foramen magnum is enlarged dorsally, which could indicate that there has been substantial bone resorbtion. Work is needed to elucidate the mechanisms of occipital growth in dogs to determine the extent to which an osteoresorbtive process can mitigate an enlarged cerebellum in CKCS and in other breeds."
However, in a June 2012 article, German researchers Martin J. Schmidt, Martin Kramer, and Nele Ondreka compared the volumes of occipital bones of cavaliers with and without syringomyelia and of French bulldogs. They did not find a reduced volume of the occipital bone of CKCSs, compared to the bulldogs. They concluded:
"These results do not support occipital hypoplasia as a cause for syringomyelia development, challenging the paraxial mesoderm insufficiency theory. This also suggests that the term Chiari-like malformation, a term derived from human studies, is not appropriate in the Cavalier King Charles spaniel."
The authors of this 2012 German article seemed mired in the pre-2010 definition of Chiari-like malformation. They state:
"... [T]he Chiari-like malformation in the Cavalier King Charles spaniel is characterized by indentation of the occipital (bone) with cerebellar herniation and is more correctly termed caudal occipital malformation syndrome."
They also appear to be unduly dismissive of the studies beginning in 2009 which found that the cavalier's cerebellum is relatively larger than that in other breeds. The authors of the 2012 German article did not include cerebellum size in their study, and their comment about the 2009-2012 reports simply is:
"Results of studies proposing a mismatch between cerebellar and caudal cranial fossa volume in this breed and in comparison to other breeds were controversial. In some studies, there was a mismatch between caudal fossa parenchyma and caudal fossa volume in dogs with syringomyelia and overcrowding was proposed as a cause of syringomyelia development. In most studies, however, no difference was found between caudal fossa volume in Cavalier King Charles spaniels with and without syringomyelia, although this was not universal." (Emphasis added.)
In a February 2013 report, UK researchers T. A. Shaw, I. M. McGonnell, C. J. Driver, C. Rusbridge, and H. A. Volk compared MRI scans of 45 CKCSs, 38 dogs of other small breeds, and 26 Labrador retrievers, and concluded:
"The data support the hypothesis that CM/SM in CKCS is a multifactorial disease process governed by the effects of increased hindbrain volume and impaired occipital bone development. The present authors recently reported that CM/SM is linked to increased cerebellar volume (Shaw and others 2012). In view of this, the aetiopathogenesis of CM/SM may equivocally be mediated by conditions independently affecting the developing occipital bones and cerebellum, or by dysregulation of a signaling mechanism coordinating the growth of the developing hindbrain and occipital skull."
In a June 2013 report, UK and German neurology researchers Joe Fenn, Martin J. Schmidt, Harriet Simpson, Colin J. Driver, and Holger A. Volk, having compared 22 cavaliers with SM and 12 without SM, found that in CKCSs with SM the percentage of space taken by venous sinuses in the brain is significantly lower than the volume occupied by the brain's parenchyma. Venous sinuses are a network of channels in the brain, which receive blood from the brains veins and also receive cerebrospinal fluid (CSF) and empty blood into the jugular vein. The report concludes that: "These results support a role for reduced venous drainage and parenchymal 'overcrowding' of the CCF [caudal cranial fossa] in the pathophysiology of SM."
In a 2013 doctorate dissertation, German Dr. Melanie Klinger studied the cranial base growth plates of 58 cavaliers and 24 other brachycephalic dogs and 67 mesocephalic dogs for the their first 18 months. She found that:
"In the CKCS the growth plate closure occurred about the 5th month of life. The second group which was composed of the brachycephalic participants of the study followed next. Finally the synchondrosis sphenooccipitalis ossificated in representatives of mesocephalic breeds around the 13.5th month."
She concluded:
"The results confirm the assumption that the premature ossification of the sphenooccipital synchondrosis is the cause of the reduced skull length for brachycephalic breeds. ... With regard to the pathogenesis of the CM the present results support the exceptional position which the CKCS possesses among the brachycephalic breeds."
See also this June 2013 article.
RETURN TO TOP
Other factors leading to SM
The severity of CM in a dog does not predict the presence of syringomyelia in that dog. Therefore, other factors are believed to influence the development of a syrinx, including atlanto-occipital overlapping (AOO) .
Ongoing research into genetic correlations between CM and SM seeks to determine whether different genes may control the expression of SM and CM. If so, it may be possible to select breeding stock which has been diagnosed to have CM but may not be expected to produce offspring with SM genes.
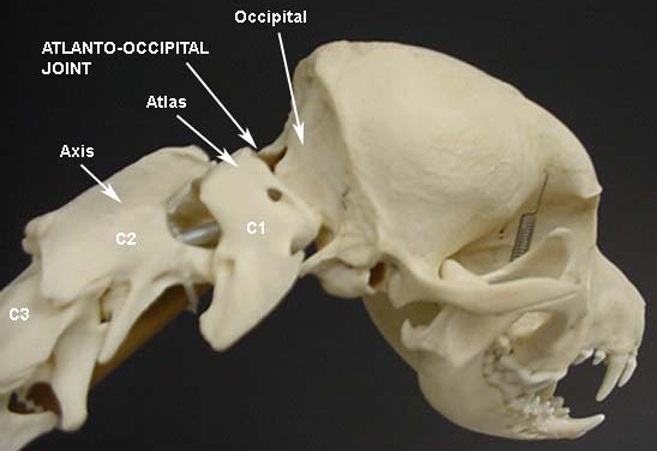
• Atlanto-Occipital Overlap (AOO)
The atlas is the spinal vertebra (C1) closest to the skull. It sits next to the foramen magnum, the hole in the occipital bone. The "atlanto-occipital joint" is the connection between the atlas and the occipital bone, and is stabilized by ligaments. "Atlantooccipital overlapping" (AOO) is characterized by a decreased distance between the atlas and the occipital bone. In some cases, the dorsal arch of the atlas may actually protrude into the foramen magnum. See the image below (courtesy of: www.wikispaces.com).
In a February 2014 article, a leading neurology team studying the Griffon Bruxellois observed:
"In other words a developmental anomaly resulting in a Chiari malformation may also be associated with abnormalities of the atlas, axis and dens. In the dog, the most important craniovertebral junction abnormality associated with CM is atlanto-occipital overlapping, which has been reported as similar to basilar invagination in humans."
In a January 2016 article, Cornell University neuroglogists examined the MRIs of 271 dogs, measuring the proximity of the atlas to the foramen magnum. They found a close association (higher than previously reported) between atlanto-occipital overlapping (AOO) and small breed dogs, including cavalier King Charles spaniels, affected with clinical signs of syringomyelia (SM).
See also these related articles: May 2009; October 2009; January 2010; April 2013; November 2015; May 2016; and June 2016.
RETURN TO TOP
Pain due to CM
See Pain due to CM under Symptoms below ...
RETURN TO TOP
Syringomyelia (SM)
- introduction to SM
- terminology of SM
- definitions of SM
- what SM is
- Role of the ventricle system
- other causes of SM
For a thorough, current review of research into syringomyelia and its
diagnosis and treatment, read Dr. Clare Rusbridge's June 2020 article, "New considerations about Chiari-like malformation,
syringomyelia and their management", linked here.
June 2020 article, "New considerations about Chiari-like malformation,
syringomyelia and their management", linked here.
Also, See also, these YouTube videos by Dr. Rusbridge that fully explain what syringomyelia is in the cavalier.
Introduction to SM
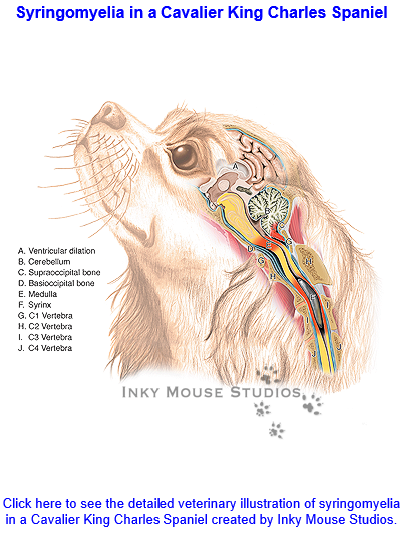 Syringomyelia
(SM) is a condition of the development of
fluid-filled cavities in the spinal cord, which is the consequence of the
shortening of the skull base --due to
brachycephaly -- and additional changes around the first vertebra,
which result in over-crowding at the junction of the brain and the
spinal cord -- the foramen magnum -- obstructing the normal free flow of
cerebrospinal fluid (CSF) between the hind end of the brain and the
spinal cord. The CSF is forced through the foramen magnum at high
pressures into the spinal cord, creating the cavities in the cord.
"Syringomyelia" is Latin for "cavity within the spinal cord".
Syringomyelia
(SM) is a condition of the development of
fluid-filled cavities in the spinal cord, which is the consequence of the
shortening of the skull base --due to
brachycephaly -- and additional changes around the first vertebra,
which result in over-crowding at the junction of the brain and the
spinal cord -- the foramen magnum -- obstructing the normal free flow of
cerebrospinal fluid (CSF) between the hind end of the brain and the
spinal cord. The CSF is forced through the foramen magnum at high
pressures into the spinal cord, creating the cavities in the cord.
"Syringomyelia" is Latin for "cavity within the spinal cord".
In the cavalier King Charles spaniel, SM is considered secondary to Canine Chiari Malformation (CM).
SM was first identified by veterinary neurologists in the 1990s, while classic symptoms, such as air scratching, had been reported anecdotally in the 1980s.
The severity and extent of syringomyelia also appear to get worse in each succeeding generation of cavaliers. Other breeds known to be affected to a lesser extent include the Affenpinscher,Bichon Frise, Boston terrier, bull terrier, French bulldog, Havanese, King Charles spaniel (the English toy spaniel), Maltese terrier, miniature dachshunds, miniature and toy poodles, Papillon, Pomeranian, Pugs, Shih Tzu, Staffordshire bull terrier, and the Yorkshire terrier. See SM in Other Breeds, below, for links to Internet articles about syringomyelia in some of these breeds.
 Courtesy of The Dog Channel
Courtesy of The Dog Channel
RETURN TO TOP
Terminology of SM
Syringomyelia is also known as syrinx and hydromyelia, and occasionally mis-identified as Arnold Chiari malformation. "Syringomyelia" is Latin for "cavity within the spinal cord".
Technically, hydromyelia is a dilatation of the central canal within the spinal cord, and syringomyelia is the cavitation of the spinal cord parenchyma. Combined, they are referred to either as syringohydromyelia (SHM) or hydro-syringomyelia. The disease is referred to generally as syringomyelia and SM herein. This condition is similar, but not identical, to Arnold Chiari Type I Syndrome in humans.
Syringomyelia also may be described as syringomyelia secondary to canine Chiari malformation (CM). CM is also referred to as occipital hypoplasia (OH) or caudal occipital malformation syndrome (COMS). The full relationship between CM and the development of SM is not fully understood. The combination of CM and SM usually is abbreviated as CM/SM.
In a
2020 article, Dr. Clare Rusbridge has explained the changes in
definitions and terminology of SM over the past two decades, as follows:
"The nomenclature of SM has morphed over the years since the first description in the early 19th century. Authoritative sources use SM rather than historical terms syringohydromyelia, hydrosyringomyelia or hydromyelia. This is because the anatomical distinction between these terms is theoretical rather than a reality (Rusbridge and Flint 2014). It is conventional in veterinary medicine to refer to a central syrinx, less than 2 mm in transverse diameter, as a central canal dilation (Fig 3), even though the ependymal lining of the central canal is disrupted with only minor dilation (Radojicic and others 2007). Non-inflammatory spinal cord oedema, as distinct from cavities containing free fluid, is referred to as presyrinx (presyringomyelia). Presyrinx most commonly affects the dorsal and ventral columns of the spinal cord and may eventually progress to SM (Fig 3). The oedema can reverse if the cause can be addressed. CNS inflammatory diseases can also cause spinal cord oedema and are alternative differentials for spinal cord oedema (Fig 4)."
RETURN TO TOP
 Definitions
of SM
Definitions
of SM
Syringomyelia (SM) is defined as "a condition that results in the development of fluid-containing cavities within the parenchyma of the spinal cord. as a consequence of abnormal cerebrospinal fluid movement." (November 2006 International Conference on Syringomyelia).
RETURN TO TOP
What SM is
Cerebrospinal fluid (CSF) surrounds the spinal cord and the brain. The brain and spinal cord literally are suspended within the CSF, which serves to insulate them from injury as they float within it. The fluid is contained in a three layered membrane called the meninges. CSF normally flows back and forth between the brain and spinal cord with each heart beat. As the heart pumps blood to the brain, the CSF flows from the brain through the hole called the foramen magnum to the spinal cord, to accommodate the increased volume of incoming blood.
Syringomyelia is believed to result when the cerebrospinal fluid is prevented from circulating normally between the brain and spinal cord, due to a narrowing or blockage of the CSF flow at the foramen magnum, thereby forcing the CSF at a higher than normal pressure into the spinal cord. called spinal cord cavitation. The pressure difference causes the spinal cord to distend or pull apart, creating a cavity called a syrinx, and squeezing fluid either from blood vessels and other tissues or CSF into the cavity. Once a syrinx is created, as the CSF moves through the spinal cord in each direction, the syrinx fills with CSF and then empties repeatedly.
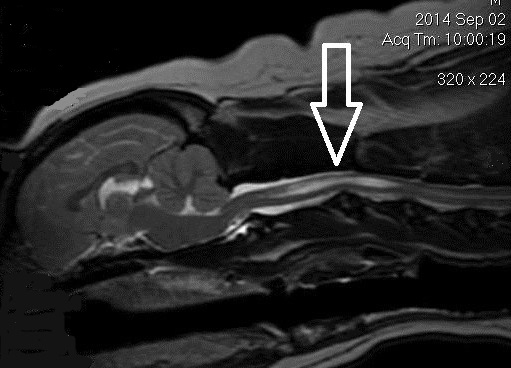
(See above a magnetic resonance imaging [MRI] scan of a
cavalier's brain and spinal cord, with the arrow
 pointing to a syrinx [the
elongated white area] within the spinal cord. The blue and gray diagram
at right -- prepared by Dr. Rusbridge -- shows the location of the syrinx
within the spinal cord. In the images of a cavalier
below, the red arrow points from a syrinx to a cross-section of the spine at
that point. This image is Figure 3 from this
November 2018 article.)
pointing to a syrinx [the
elongated white area] within the spinal cord. The blue and gray diagram
at right -- prepared by Dr. Rusbridge -- shows the location of the syrinx
within the spinal cord. In the images of a cavalier
below, the red arrow points from a syrinx to a cross-section of the spine at
that point. This image is Figure 3 from this
November 2018 article.)
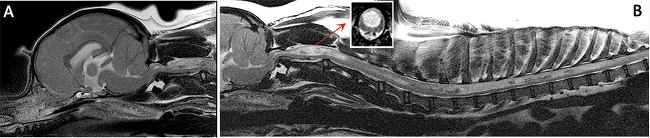
However, in a September 2015 abstract before the ESVN-ECVN, UK researchers created a computer model of the spinal cord, subarachnoid space (SAS), dura mater, and the epidural space of a cavalier King Charles spaniel affected with CM/SM. They performed exaggerated movement of the spinal cord during the cardiac cycle, seeking to confirm a theory that abnormities in the circulation of the cerebrospinal fluid (CSF) generate pressures that drive the fluid into the cord. Instead, they found that CSF pressure gradients are unlikely to cause fluid movement into the cord, sufficient to generate syrinxes. They concluded:
"On the other hand, although the shear stress in the cord is low, its location and cyclic nature indicates the possibility that this may be the factor that generates the initial tissue damage, which eventually leads to the formation of syrinxes."
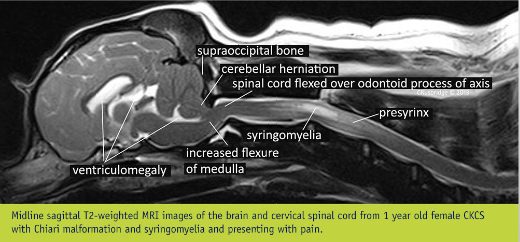
Syringomyelia is an extremely serious, progressively worsening spinal disease which is rare in most breeds but is becoming very widespread in cavalier King Charles spaniels of all bloodlines. In May 2005, Dr. Rusbridge and Susan P. (Penny) Knowler, BSc (Hons), who have been studying the disease in several hundreds of cavaliers, reported that a conservative estimate is that at least 50% of cavalier King Charles spaniels have a degree of Chiari-like malformation, although not all are so severely affected as to have syringomyelia. In February 2010, Dr. Georgina Child, board certified veterinary neurologist in Australia, reported that of 60 asymptomatic cavaliers scanned as potential breeding stock, 50% had SM syrinxes. In a September 2010 report of 804 cavaliers, Mrs. Knowler and others estimated that "the lifetime risk of developing syringomyelia in the study population was estimated to be 55%."
In a 2011 study of 49 cavaliers diagnosed with SM, Dr. Rusbridge and others found that "total syrinx size was positively correlated with age" of the dogs. In a June 2011 study of 555 cavaliers without any symptoms of syringomyelia, 25% of the one year old dogs had SM and 70% of the dogs aged 6 years and older had SM.
In an
October 2021 article, Drs. Srdjan Cirovic and Clare Rusbridge report
on a computer model they devised
 which may explain how SM syrinxes (syringes) are created and expand, due to the impulsive movement of
cerebrospinal fluid (CSF), called "slosh". A magnetic resonance imaging
(MRI) scan of a cavalier was used as the model
design for the study. The investigators conducted simulations of various
spinal cord conditions, from the cord being free of cavities to small
syringes at different locations to a prorgessively expanding syrinx.
(See Figure 2.)
They found that, if small syringes are present, there are peaks of
stress at those locations, the effect being most pronounced at the
locations at which syringes initially form. When the syrinx reaches the
lumbar region, the stress becomes moderate. They concluded that their
findings support the "slosh" hypothesis, suggesting that small cervical
syringes may progress, but when the syrinx is large, there is less
stress, which may explain why a syrinx can rapidly expand but then
remain unchanged in shape over years. Their stated conclusions:
which may explain how SM syrinxes (syringes) are created and expand, due to the impulsive movement of
cerebrospinal fluid (CSF), called "slosh". A magnetic resonance imaging
(MRI) scan of a cavalier was used as the model
design for the study. The investigators conducted simulations of various
spinal cord conditions, from the cord being free of cavities to small
syringes at different locations to a prorgessively expanding syrinx.
(See Figure 2.)
They found that, if small syringes are present, there are peaks of
stress at those locations, the effect being most pronounced at the
locations at which syringes initially form. When the syrinx reaches the
lumbar region, the stress becomes moderate. They concluded that their
findings support the "slosh" hypothesis, suggesting that small cervical
syringes may progress, but when the syrinx is large, there is less
stress, which may explain why a syrinx can rapidly expand but then
remain unchanged in shape over years. Their stated conclusions:
"The results of this study strongly suggest that the spinal cord tissue in the vicinity of fluid-filled cavities experiences higher than normal mechanical stress due to the movement of the CSF from epidural excitation. When the syringes are longer than approximately 30 mm, filling of the epidural veins may generate the "slosh" effect, where the fluid is forced to the caudal end of the syrinx. The results for the simulations of an expanding syrinx are broadly consistent with the homeostatic hypothesis, as the stress in the cord is lower for the fully developed syrinx than for smaller syringes. Other, potentially more realistic, scenarios for syrinx expansion should be examined in the future. This study specifically addresses syringomyelia in dogs, and more specifically in CKCS. ... Considering anatomical and other differences (e.g., upright posture in humans) the results regarding the potential pattern of syrinx enlargement do not apply to humans or to dog breeds other than CKCS."
RETURN TO TOP
Role of the ventricle system (ventricular dilatation - ventriculomegaly)
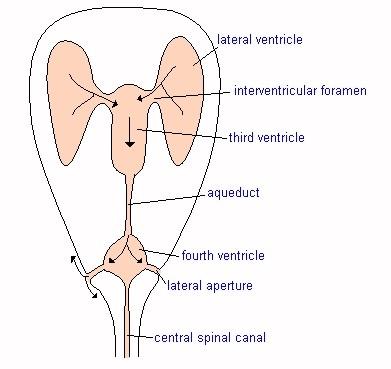 The brain's ventricle system consists of
four cavities which are connected with the spinal cord's central canal. The
four ventricles are known as the two lateral ventricles, the third
ventricle, and the fourth ventricle. The ventricles are the source of CSF
and are the brain's respository of CSF.
The brain's ventricle system consists of
four cavities which are connected with the spinal cord's central canal. The
four ventricles are known as the two lateral ventricles, the third
ventricle, and the fourth ventricle. The ventricles are the source of CSF
and are the brain's respository of CSF.
Some neurologists are including in their examination reports an analysis of whether the ventricles are dilated, and if so, a measurement of the amount of their dilatation. The medical term for dilated lateral ventricles is ventriculomegaly. (See our ventriculomegaly webpage for more information about this disorder.)
In a December 2010 UK study led by Dr. Colin Driver, , the researchers' results were consistent with the previous findings that ventriculomegaly and a small but significant increase in caudal fossa parenchyma are associated with syringomyelia.
In an October 2016 abstract, German researchers compared the perfusion of blood in the periventricular white matter of 23 cavalier King Charles spaniels with ventriculomegaly compared to control dogs consisting of 10 healthy Beagles. They found that cerebral blood flow and volume were significantly lower in the cavaliers. They concluded that the dogs with ventriculomegaly may have a form of normal pressure hydrocephalus (NPH).
In an August 2017 article, the same German team studied the ventricle system of 42 cavaliers -- 32 CKSCs with ventriculomegaly and 10 control CKCSs. They used "dynamic susceptibility contrast perfusion" magnetic resonance imaging (DSC-PMRI), which allows them to quantify the volume of blood passing through the brain tissue. They found that cerebral blood flow (CBF) is reduced in the periventricular white matter of CKCSs with ventriculomegaly, which makes some increase of intraventricular pressure likely. They stated that these findings make it plausible that ventriculomegaly may be a form of internal hydrocephalus. They observed that when intraventricular pressure is increased, a higher cerebrospinal fluid (CSF) volume is forced from the ventricles at a higher velocity.
In a July 2022 article, Italian researchers reviewed the clinical records of 43 cavaliers diagnosed with CM, to to calculate the sizes of their lateral ventricles and determine if there is any the association between ventriculomegaly and clinical signs, ventricular asymmetry, grade of CM, SM, and degree of medullary kinking. The most common initial clinical signs were scratching and neck pain. Ventriculomegaly was identified in 70% of dogs, CM grade 2 (CM2) was observed in 77% of cases, ventricular asymmetry in 54% and SM in 80% of the cavaliers. The median medullary kinking index was 37.77%, and 28% of the dogs had epileptic seizures. They report finding:
• No significant association between the grade of SM and class of ventriculomegaly.
• No significant association between dimension of lateral ventricles and any other conditions.
• No significant association between medullary kinking index and class of ventriculomegaly.
• No significant association between primary secretory otitis media (PSOM) and any clinical signs.
They concluded that "the prevalence of ventriculomegaly in Cavalier King Charles Spaniels is high but this finding does not seem related to the severity of clinical signs, presence of Chiari-like malformation, syringomyelia and craniocervical junction abnormalities such as medullary kinking.
Below is a comparison between a canine brain with normal lateral cerebral ventricles (A) and one with enlarged lateral ventricles (B). From a May 2015 study led by Dr. Martin J. Schmidt.
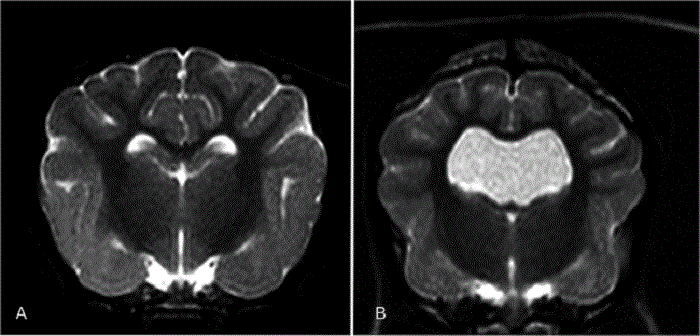
RETURN TO TOP
Other causes of SM
There are other forms of syringomyelia in canines. Spinal dysraphism or spinal dysplasia is a genetic disorder in which puppies normally under the age of three months display a bunny hopping gait and wide-based stance and scoliosis, due to the spinal cord not developing completely in the womb. Dalmatians, English setters, golden retrievers, rottweilers, visla, and Weimaraners have been identified with this disorder. Also, SM may be caused by tumors, cysts, or trauma. Neither of those are discussed here.
Also, benign, small syrinxes are a common incidental finding on MRI
examinations. Therefore, clinical
correlation is important in order
to determine if the syrinx is associated with the Chiari-like
malformation and/or symptoms.
RETURN TO TOP
Symptoms
The following paragraphs discuss typical symptoms (clinical and behavioral signs) displayed by dogs diagnosed with Chiari-like malformation (CM) and/or syringomyelia (SM). CM and SM each cause different symptoms. Most signs of CM are due to pain. SM also can cause pain, but usually only late in the progression or in the most severe cases. The most common symptom of SM is phantom scratching. For more information about pain due to CM and SM, see Expressions of Pain, below.
Several of these signs are so common that they may be for other reasons and unrelated to CM or SM at all. For example, prior studies have shown that approximately 25% of dogs that display clinical signs of SM are not found to have a syrinx on MRI scans. Scroll down to "Other disorders with similar symptoms" for a discussion of likely other disorders causing similar signs.
SM and CM very seldom can be detected in young puppies, as symptoms usually are not evident before the age of six months or even many years later. There is no way to know in advance of the symptoms whether a dog is normal or is a syringomyelia carrier which does not develop the disease but can pass it on to its offspring.
NOTE: Veterinarians will tell us that dogs do not have "symptoms" but instead have "signs". But, for us laymen, the word "signs" can be confusing because of its different meanings. So, for us, "symptoms" it is.)
Symptoms due to CM
 The most common symptoms of Chiari-like malformation (CM) are
the result of feeling head aching pain, such as grimacing facial
expressions (pain face) and squinting at bright lights (photophobia).
Other indications include:
The most common symptoms of Chiari-like malformation (CM) are
the result of feeling head aching pain, such as grimacing facial
expressions (pain face) and squinting at bright lights (photophobia).
Other indications include:
• Vocalization in 65% of cavaliers diagnosed with CM: Yelping or moaning when the dog changes its position, or when being lifted up, or while asleep, or seemingly at random.
• Head (especially at the back of the head) and ear scratching or rubbing the face on surfaces, in 28% of cavaliers diagnosed with CM. See Dr. Clare Rusbridge's short YouTube video demonstrating this type of scratching and rubbing.
This section discusses typical symptoms (clinical and behavioral signs) displayed by dogs diagnosed with CM. Most signs of CM are due to pain. SM also can cause pain, but usually only late in the progression or in the most severe cases. (See "Pain Due to SM", below.)
Symptoms, signs, and behaviors which may be due to CM include:
• Expressions of pain, discussed below
• Temderness at the neck, shoulders, and/or head
• Head shaking (CM or SM)
• Lip licking
• Head rubbing -- side to side on the floor
• Reduced physical activity (CM or SM)
• Behavioral change to more timid, anxious, or aggressive
• Touch aversion (CM of SM)
• Inability to lower head to eat or drink (CM or SM)
• Restlessness -- inability to settle down
• Sleep disorders
It also is possible that a dog with CM does not have SM but still may have symptoms of SM due to the CM obstructing the flow of cerebrospinal fluid (CSF). This also is attributed to a direct compression of the medulla oblongata, which is involved in the modulation of pain.
In a November 2018 article, Drs. Rusbridge and Knowler summarize how to diagnose pain due to CM as follows:
"Diagnosis of CM-pain is made by appropriate clinical signs in addition to MRI brain findings of a brachycephaly with rostrotentorial crowding including rostral flattening, olfactory bulb reduction and rotation, increased height of the cranium with reduction of the functional caudotentorial space and hindbrain herniation. There may also be changes suggesting raised intracranial pressure such as loss of sulci definition with ventriculomegaly. The cisterna magna is reduced."
In an October 2012 study by UK researchers of 48 cavaliers, nine of which had only CM and the rest also had SM, neuropathic pain progressed in 75% of the dogs over a mean average period of 39 months. The researchers noted that it is not fully understood how CM/SM causes neuropathic pain, and they did not make any such finding. However, their report confirms that neuropathic pain does exist, and it progresses, in cavaliers with only CM.
In a January 2017 article, UK researchers determined, from its group of 28 cavaliers with pain due to CM but not SM, that they "had a short basicranium (line ab in Figure 1 below) with a resultant compensatory increased cranial height (small angle 7, below) and increased brachycephaly with olfactory bulb more ventrally rotated (p = 0.003) and rostral forebrain flattening compared to Control CKCS. However, in comparison with SM dogs, the CM cohort has a longer line bc and a wider angle 9 increases the volume of the caudal fossa, which may lessen obstruction to CSF flow and the risk of developing SM."
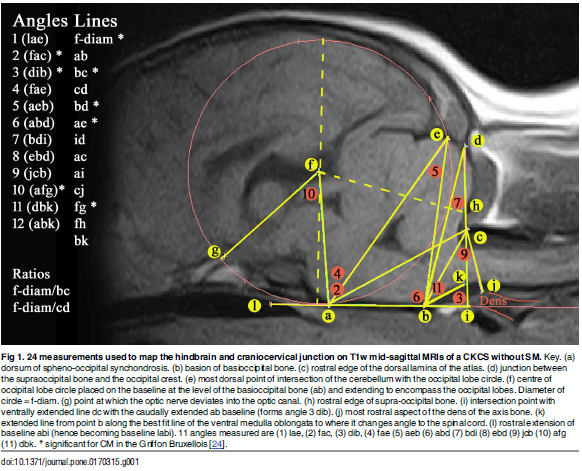
In a November 2017 article, a team of researchers at NC State University and Cornell University prepared and studied the results of a questionnaire answered by owners of 50 cavalier King Charles spaniels, 20 with only Chiari-like malformation (CM) and 30 with both CM and syringomyelia (SM). Of the 50 dogs, 33 were symptomatic and 17 were not. The most common presenting sign was phantom scratching, occurring in 32 dogs, and the most common sign of pain was crying out when being lifted, occurring in 11 dogs. The researchers found that owner-reported findings were not significantly associated with presence or severity of SM or neurologic examination findings. Ten of the 20 CM-only dogs reportedly displayed "classic" signs of neuropathic pain.
The series of questionnaires are linked here: Preliminary Questionnaire -- Dog Diagram (ChiMPS-M) -- Final Questionnaire (ChiMPS-T). The researchers concluded:
"T" The conclusion that SM causes pain in CKCS is complicated by the finding that dogs with CM but no SM can show classic signs of neuropathic pain, as illustrated by 10 dogs in our study. ... To conclude, the full range of signs reported by owners of CKCS includes a variety of manifestations of pain, with phantom scratching as the most commonly reported sign followed by crying out when being lifted. Owner reporting of pain and scratch frequency and severity captured by the ChiMPS-T correlates with the owner-reported surface area affected by these signs in their dogs. Neither the scores nor the surface area reported correlated with the presence or severity of SM, highlighting uncertainty on the source of pain in these dogs."
In Figure 8 below, this comparison series of MRI scans of three cavaliers (from this November 2018 article by Drs. Rusbridge and Knowler) demonstrates differences in the positioning (and crowding) of the brain among dogs with normal, CM-pain-affected, and SM-affected conditions. See, also, our discussion of Syringomyelia -- Expressions of Pain, below.
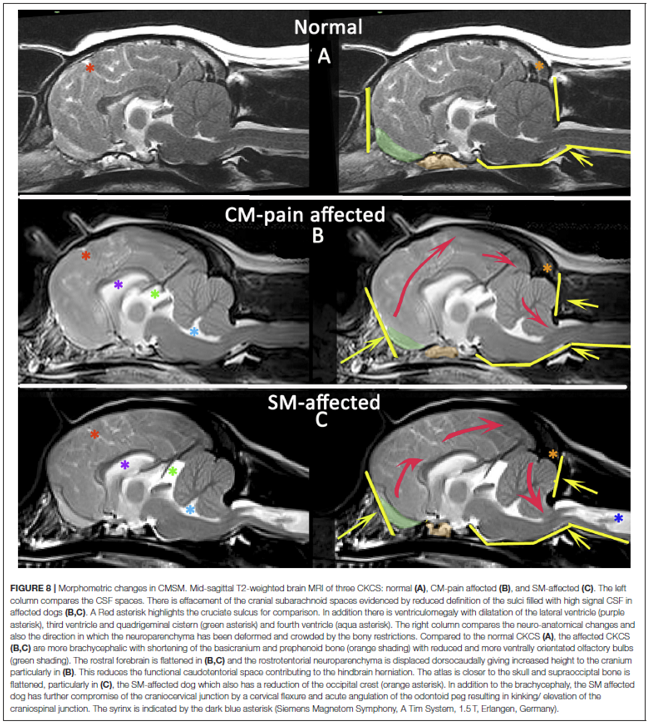
In a July 2019 article, UK neurology researchers studied the records of 130 cavalier King Charles spaniels diagnosed with CM and some also with SM to determine which symptoms (clinical and behavioral signs) related to CM and to SM and to syrinx diameter, in order to use the data in future studies of diagnosis, treatment, and genetics of CM/SM. Dogs were grouped based upon whether they had no SM (Group 1) up to having a syrinx greater than 4 mm (Group 4).
Signs found NOT RELATED to syrinx presence or size:
• Vocalization (65.4%) (except being picked up under the sternum (breastbone), which was more common among dogs with no or mild SM).
• Spinal pain -- 54.6%
• Reduced activity -- 37.7%
• Reluctance to jump or to climb stairs --35.4%
• Aversion to touch or grooming -- 30.0% (ears, head, and neck region -- 25.4%)
• Change in emotional state (more timid, anxious, withdrawn, or aggressive) -- 28.5%
• Disrupted sleep -- 22.3%
The implication from this study is that the major signs of pain expressed by CM/SM cavaliers definitely are not due to having a syrinx and probably are due to having Chiari-like malformation, but the authors cannot definitely attribute the painful expressions solely to CM.
 As the disorder progresses, there usually follows increasingly severe
pain around the dog's head, neck, and shoulders, causing it yelp or scream.
This is described as "vocalization" -- spontaneous, when picked up under the
sternum, or when changing position especially at night.
It is believed to be a neuropathic pain, probably due to disordered neural
processing in the damaged dorsal horn.
As the disorder progresses, there usually follows increasingly severe
pain around the dog's head, neck, and shoulders, causing it yelp or scream.
This is described as "vocalization" -- spontaneous, when picked up under the
sternum, or when changing position especially at night.
It is believed to be a neuropathic pain, probably due to disordered neural
processing in the damaged dorsal horn.
(Photos at right are of a cavalier both without pain and then suffering severe head pain due to CM. Courtesy of Dr. Clare Rusbridge, Fitzpatrick Referrals, UK.)
In a May 2014 report, USA researchers assessed 36 cavalier King Charles spaniels for neurologic pain and dysfunction. They found that 20 of the dogs demonstrated neuropathic pain; that dural bands (compressive lesions caused by abnormally thickened dura mater* at the craniocervical junction) were present in 31 of the dogs; that 34 of the dogs had Chiari-like malformation; that 23 of the dogs had syringomyelia (and 21 of those 23 dogs had dural bands). They also found that dural bands were associated with both the presence and severity of clinical signs and the presence of SM, and that higher compression indices were associated with more severe SM. They concluded that:
"Dural bands appear to play a significant clinical role. Compression indices provide a better assessment of dural band severity compared to grading."
* Dura mater is is a thick membrane made of dense tissue that surrounds the brain and spinal cord. It is the outermost of the three layers of membrane called the meninges that is directly inside the skull bone.
Similar forms of neuropathic pain in humans suffering from Chiari type I malformation (the human counterpart to CM and SM include: (a) a burning type pain, pins-and-needles and other odd sensations (called dysaesthesia); (b) pain from a stimulus which is not normally painful, such as light touch or motion (called allodynia); (c) increased pain from stimuli which are normally painful (called hyperpathia,); and (d) a constant, burning type pain (called causalgia). In humans, neuropathic pain also is associated with anxiety, depression, and reduced quality of life.
In a January 2025 YouTube video, Dr. Clare Rusbridge observed that as CM-affected cavaliers grow older, their brains shrink, and as a consequence, the severity of fhe CM -- and thus the resulting pain -- may reduce.
RETURN TO TOP
Symptoms due to SM
- Phantom scratching
- Other common symptoms
- Expressions of pain
- Changes in barometric pressure
- Other disorders with similar symptoms
- Symptoms not due to CM/SM
The most common symptom of syringomyelia (SM) is phantom scratching. In 48% of cavaliers diagnosed with SM, they display rhytmic scratching with a rear leg, where the foot scratches at empty air, usually when the dog is being walked on a leash. See Dr. Clare Rusbridge's short YouTube video demonstrating this type of scratching.
The following paragraphs discuss typical symptoms (clinical and behavioral signs) displayed by dogs diagnosed with SM. CM and SM each cause different symptoms. Most signs of CM are due to pain. (See "Pain Due To CM", below.) SM also can cause pain, but usually only late in the progression or in the most severe cases. (See "Pain Due to SM", below.)
Several of these signs are so common that they may be for other reasons and unrelated to CM or SM at all. For example, prior studies have shown that approximately 25% of dogs that display clinical signs of SM are not found to have a syrinx on MRI scans. Scroll down to "Other disorders with similar symptoms" for a discussion of likely other disorders causing similar signs.
Phantom scratching
 •
What it is
•
What it is
Symptoms of CM and SM may vary widely among different dogs, but the most common sign of SM (and not of CM) often is that the dog feels a sensitivity in its neck area, causing in some an uncontrollable urge to scratch at its neck and shoulders excessively, particularly when walking or during other forms of exercise, and usually without making skin contact. It also can be prompted by rubbing certain areas (see dermatome, below) of the skin. This is called phantom scratching or fictive scratching or maladaptive scratching. See the description of phantom scratching by Dr. Clare Rusbridge in her YouTube video linked here.
• What it is not
Phantom scratching is specifically limited towards the neck area and in most all cases, only on one side. Chronic scratching in other areas is not the phantom scratching associated with SM. For example, dogs with CM may scratch at the back of their head or their ears due to head pain. In general, head or ear scratching, or being itchy all over the body, is not phantom scratching nor is it related to CM. (Photo at right is courtesy of Passionate Productions.) See also, the description of phantom scratching by Dr. Clare Rusbridge in her YouTube video linked here.
In a July 2019 article, a team of UK neurology researchers studied the records of 130 cavalier King Charles spaniels diagnosed with CM and some also with SM to determine which symptoms related to CM and to SM and to syrinx diameter, in order to use the data in future studies of diagnosis, treatment, and genetics of CM/SM. Dogs were grouped based upon whether they had no SM (Group 1) up to having a syrinx greater than 4 mm (Group 4).
Signs found NOT RELATED to syrinx presence or size:
• Vocalization (65.4%) (except being picked up under the sternum (breastbone), which was more common among dogs with no or mild SM).
• Spinal pain -- 54.6%
• Reduced activity -- 37.7%
• Reluctance to jump or to climb stairs --35.4%
• Aversion to touch or grooming -- 30.0% (ears, head, and neck region -- 25.4%)
• Change in emotional state (more timid, anxious, withdrawn, or aggressive) -- 28.5%
• Disrupted sleep -- 22.3%
Signs found TO BE RELATED to syrinx presence or size:
• Phantom scratching -- 67% of Group 4 dogs and none in other groups
• Scratching or rubbing of the head or ears -- 28% but less common in Group 4 dogs
• Scoliosis -- 27% of Group 4 dogs and none in other groups
• Postural defects -- 15% of Group 4 dogs and none in other groups
• Weakness -- 39% of Group 4 dogs and none in other groups
The researchers stated that their findings suggest that "phantom scratching is highly unlikely with small syrinxes." They also found that among the dogs in their study, PSOM (primary secretory otitis media) is common in symptomatic CM-affected dogs and in SM-affected dogs, thereby raising the possibility of confusing the cause of some signs and behaviors, since PSOM-affected dogs tend to engage in ear-rubbing. They concluded:
"The study further suggests that SM-specific signs are phantom scratching, scoliosis, and sensory and motor signs that can be related to spinal cord damage by the syrinx and are associated with large syringes (transverse width >4 mm). Non-SM-specific signs include vocalization (described as without obvious trigger, when shifting position when recumbent and when being lifted under the sternum to a height), spinal pain, head and ear rubbing or scratching, aversion to touch, refusal or difficulty jumping or doing stairs, exercise intolerance/reduced activity, sleep disruption, or behavioral change described as becoming more anxious, timid, aggressive, or withdrawn. These non-SM-specific signs could reflect CM-P [CM associated pain]."
The implication from this study is, therefore, that the major signs of pain expressed by CM/SM cavaliers definitely are not due to having a syrinx and probably are due to having Chiari-like malformation, but the authors cannot definitely attribute the painful expressions solely to CM.
• Dorsal horn damage as cause
In Dr. Rusbridge's January 2024 YouTube video on phantom scratching, she states that being able to prompt or evoke phantom scratching by rubbing an area of the dog's skin indicates that there is dorsal horn damage caused by a syrnix. The specific area of the skin is a dermatome, which is an area of skin receiving sensory innervation from a single spinal nerve dorsal root. The location of the syrinx causing damage to the dorsal horn is at the C2-C4 vertebrae, particularly if the syrinx wide and is asymmetrical and extends up to the dorsal nerve root entry zone. Occasionally a syrnix in the cranial thoracic region will cause scratching near the sternum (breastbone).
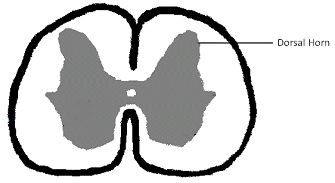 Dorsal horns are two projections from the rear of the spinal column
which contain neurons and cells of the central nervous system. (See
diagram at right. They are at the outer tips at the 2 o'clock and 10
o'clock locations.) Each of the two horns consists of layers of gray
matter called "laminae", which are divided into sections (laminae I
through VI in the dorsal horn) based upon their location and function.
Dorsal horns are two projections from the rear of the spinal column
which contain neurons and cells of the central nervous system. (See
diagram at right. They are at the outer tips at the 2 o'clock and 10
o'clock locations.) Each of the two horns consists of layers of gray
matter called "laminae", which are divided into sections (laminae I
through VI in the dorsal horn) based upon their location and function.
In a March 2022 article, Danish researchers studied eight cavaliers diagnosed with SM and four others without SM, to determine if phantom scratching on only one side of the body of SM-affected dogs related to pathological changes in their spinal cords. Seven of the eight SM-affected CKCSs displayed signs of pain by phantom scratching on only one side of their bodies, called "unilateral scratching". They examined the two dorsal horns in spinal cord segments C1 through C8 specifically. The investigators reported finding that the cavaliers which phantom scratched on only one side had some loss of the volume of laminae I through III of the dorsal horn which is located on the same side of the spinal cord as the side the dog phantom scratches, compared to the dorsal horn on the opposite side. They also noted that in cavaliers which unilaterally scratched, there were changes in the pain pathways at the dorsal root entry zone of those dogs.
• Prior research into causes
Phantom scratching previously had been believed to be due to an increase in the pressure of the flow of cerebrospinal fluid through the central canal from the brain down the spinal column, causing the central canal to expand and press against the nerves of the spinal column and creating a pins-and-needle-like tingling or a burning-type pain, and other strange sensations (called dysaesthesia or paresthesia), which prompt the dog to scratch.
However, in a
May 2016 abstract, UK researchers fou nd that phantom scratching is
associated with a very wide syrinx -- 4 mm. wide or greater in cavaliers -- that extends to the superficial dorsal horn (SDH)
in the C3-C6 spinal segments. The study found that phantom scratching is
associated with a large dorso-lateral syrinx
 that extends to the SDH in the
C3-C6 spinal segments (C2-C5 vertebrae). The study did not find an association
to damage of other areas of cervical spinal cord. They suggested that phantom
scratching is due to damage to projection neurons in lamina I of the superficial
dorsal horn (SDH) with resulting reduced descending inhibition to the
lumbosacral scratching CPG [central pattern generator -- neural circuits
controlling a stereotyped sequence of muscle contractions]. ... They concluded
that if a dog has an SM syrinx extending to the SDH then it is at risk for
phantom scratching. In a
November 2017 article -- an extended publication of the
May 2016
abstract -- the same investigators concluded:
that extends to the SDH in the
C3-C6 spinal segments (C2-C5 vertebrae). The study did not find an association
to damage of other areas of cervical spinal cord. They suggested that phantom
scratching is due to damage to projection neurons in lamina I of the superficial
dorsal horn (SDH) with resulting reduced descending inhibition to the
lumbosacral scratching CPG [central pattern generator -- neural circuits
controlling a stereotyped sequence of muscle contractions]. ... They concluded
that if a dog has an SM syrinx extending to the SDH then it is at risk for
phantom scratching. In a
November 2017 article -- an extended publication of the
May 2016
abstract -- the same investigators concluded:
"SM associated phantom scratching appears associated with MRI findings of a large syrinx extending into the mid cervical SDH. We hypothesise that damage in this region might influence the lumbosacral scratching central pattern generator (CPG). If a scratching SM affected dog does not have a large dorsolateral cervical syrinx with SDH involvement then alternative explanations for scratching should be investigated."
This article suggests that only dogs with both CM and SM will phantom scratch, and that dogs with only CM will not. It also suggests that the action is very similar to fictive scratching which occurs in animals with severed spinal cords.
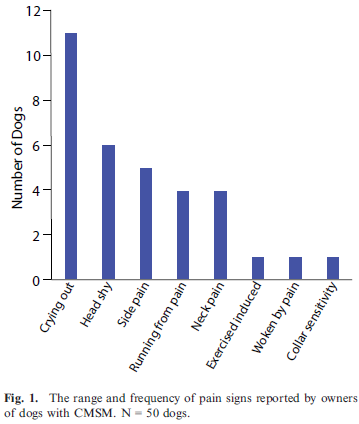 In a
November 2017 article,
a team of researchers at NC State University and Cornell University prepared and studied the
results of a questionnaire answered by owners of 50 cavalier King
Charles spaniels, 20 with only Chiari-like malformation (CM) and 30 with
both CM and syringomyelia (SM). Of the 50 dogs, 33 were symptomatic and
17 were not. The most common presenting sign was phantom scratching,
occurring in 32 dogs, and the most common sign of pain was crying out
when being lifted, occurring in 11 dogs. The researchers found that
owner-reported findings were not significantly associated with presence
or severity of SM or neurologic examination findings. See Figure 1
(right), a chart of the symptoms reported by the owners of the 33
symptomatic dogs.
In a
November 2017 article,
a team of researchers at NC State University and Cornell University prepared and studied the
results of a questionnaire answered by owners of 50 cavalier King
Charles spaniels, 20 with only Chiari-like malformation (CM) and 30 with
both CM and syringomyelia (SM). Of the 50 dogs, 33 were symptomatic and
17 were not. The most common presenting sign was phantom scratching,
occurring in 32 dogs, and the most common sign of pain was crying out
when being lifted, occurring in 11 dogs. The researchers found that
owner-reported findings were not significantly associated with presence
or severity of SM or neurologic examination findings. See Figure 1
(right), a chart of the symptoms reported by the owners of the 33
symptomatic dogs.
Ten of the 20 CM-only dogs reportedly displayed "classic" signs of neuropathic pain. The researchers concluded:
"The conclusion that SM causes pain in CKCS is complicated by the finding that dogs with CM but no SM can show classic signs of neuropathic pain, as illustrated by 10 dogs in our study. ... To conclude, the full range of signs reported by owners of CKCS includes a variety of manifestations of pain, with phantom scratching as the most commonly reported sign followed by crying out when being lifted. Owner reporting of pain and scratch frequency and severity captured by the ChiMPS-T correlates with the owner-reported surface area affected by these signs in their dogs. Neither the scores nor the surface area reported correlated with the presence or severity of SM, highlighting uncertainty on the source of pain in these dogs."
RETURN TO TOP
Other common symptoms of SM
 Some dogs perform facial or head rubbing or spontaneous vocalizations.
Click here
or the YouTube logo (right) to see videos of cavaliers with SM
symptoms. Videos also are available under Related Links
below.
Some dogs perform facial or head rubbing or spontaneous vocalizations.
Click here
or the YouTube logo (right) to see videos of cavaliers with SM
symptoms. Videos also are available under Related Links
below.
Symptoms, signs, and behaviors which may be due to SM include:
• Phantom scratching, discussed above
• Scratching of both sides of the neck and shoulders
• Aversion to being touched to the head, neck, or shoulders
• Worsening of clinical signe when the dog was emotionally aroused
• Preferred head posture during sleep
• Pain, usually late in the progression or in the most severe cases. (See "Pain Due to SM", below.)
• Head shaking
• Feet licking or chewing -- excessive
• Reduced physical activity
• Inability to lower head to eat or drink
• Weakness of the hind limbs
• Stiffness of the limbs
Many symptoms of SM, such as scratching, are so ordinary (when not excessive or compulsive) that they could be attributed to any of several common causes, including flea bites or allergies. Others, such as limping or lack of muscle coordination also could be confused with injuries or other disorders.
Scoliosis (abnormal curving of the spine) is another physically apparent symptom. (See photo of a young cavalier with scoliosis, below.) Dr. Rusbridge reports finding that scoliosis in many SM-affected dogs will improve slowly over the years.

An outward deviation of an eye, called exotropia, also is common among dogs affected with CM.
SM and CM very seldom can be detected in young puppies, as symptoms usually are not evident before the age of six months or even many years later. There is no way to know in advance of the symptoms whether a dog is normal or is a syringomyelia carrier which does not develop the disease but can pass it on to its offspring.
SM causes damage to the spinal cord and usually results in symptoms of hypersensitivity, intense pain, and leg dysfunction. The primary symptoms may vary widely, and in some cases, a cavalier may even have SM without displaying any outward symptoms at all. Some cavaliers diagnosed with SM lack any clinical signs. It also is possible that a dog with CM does not have syringomyelia (the syrinx in the spinal cord), but still may have symptoms of SM due to the CM obstructing the flow of cerebrospinal fluid (CSF). This also is attributed to a direct compression of the medulla oblongata, which is involved in the modulation of pain.
In the absence of an MRI, the severity of certain SM-related symptoms and their combinations have been found to indicate the size range of syrinxes in cavaliers. In a February 2024 article, Danish researchers investigated the symptoms of 89 cavaliers which had been diagnosed with SM by MRI at the University of Copenhagen, Denmark. They interviewed the dogs' owners, to determine the relationship between the sizes of the dogs' syrinxes and their SM-related symptoms. The severity of the dogs' CM and SM were cagtegorized by the British Veterinary Association/Kennel Club scheme of measuring the condition of the CM and sizes of the syrinxes. The dogs were distributed into three groups: (1) dogs with CM and no SM (or with a maximum transverse width < 2 mm), (2) dogs with CM and small syrinx (SM 2.00-3.99 mm) and (3) dogs with CM and large syrinx (SM >4 mm). They report finding that these clinical signs were reported significantly more frequently in dogs with large syrinxes:
• Phantom scratching
• Scratching of both sides of the neck and shoulders
• Aversion to being touched to the head, neck, or shoulders
• Worsening of clinical signe when the dog was emotionally aroused
• Preferred head posture during sleep
They found that when phantom scratching, aversion to touch, and a preferred head posture while asleep were combined, the likelihood of a large syrinx increased. They concluded that clinicians may use this information to diagnose CKCSs with a large syrinx and necessary treatment when MRI diagnosis is not available or affordable.
 Ease
the symptoms by using a comfortable harness instead of a collar and
leash. One of the best harnesses for cavaliers with CM/SM symptoms
is the BRILLIANT K9 "Lucy Small" harness,
available on Amazon.
It is easy to put on and easy to take off. Watch the videos:
"Opening the harness" and
"Walking the dog with the harness".
Ease
the symptoms by using a comfortable harness instead of a collar and
leash. One of the best harnesses for cavaliers with CM/SM symptoms
is the BRILLIANT K9 "Lucy Small" harness,
available on Amazon.
It is easy to put on and easy to take off. Watch the videos:
"Opening the harness" and
"Walking the dog with the harness".
RETURN TO TOP
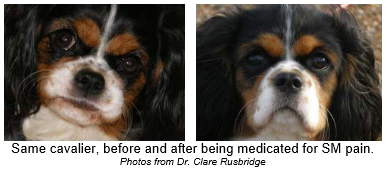 Expressions of pain
Expressions of pain
As syringomyelia destroys portions of the cavalier's spinal cord, the dog may experience so much pain that it may contort its neck and may even sleep and eat only with its head held high. Ultimately, the dog may develop scoliosis, as a result. There may also be progressive weakness in the legs, so that walking becomes increasingly difficult. Some dogs deteriorate to the point of paralysis.
In a June 2007 study of 55 cavaliers, the researchers reported that the wider the syrinx, the stronger the predictor of pain, scratching behavior and scoliosis in dogs with syringomyelia. They stated: "Both pain and syrinx size were positively correlated with syrinxes located in the dorsal half of the spinal cord." They also concluded that such pain is likely to be neuropathic pain, resulting from disordered neural processing in the damaged dorsal horn. Similarly, in an August 2012 study, the researchers found evidence that:
"... the disruption of the dorsal horn structure is a significant event in the production of clinical signs in CKCS. The spinal cord dorsal horn in symptomatic CKCS is significantly more asymmetric than that of control animals, whereas the asymptomatic CKCS have changes that are midway between control and symptomatic CKCS. This suggests the possibility that progression from mild to severe asymmetry in CKCS is associated with development of clinical signs; however such a conclusion cannot be definitively supported by this study because of the cross sectional nature of the data collected."
 Syringomyelia can be very deceptive because some symptoms (which may
include paw licking, head shaking, head rubbing, circular walking, fly
biting, and reluctance to defecate) are common behaviors for many unaffected
dogs. One distinction is that dogs suffering from SM engage in these
patterns excessively and seemingly compulsively. So, other causes of the
dog's symptoms need to be considered and should be ruled out before
concluding that SM is the cause. For example, if a syrinx develops in a
lower area of the spine, such as the lumbar region, the dog may scoot
excessively, even to the extent of rubbing the anal area raw. However,
scooting is a common symptom of other disorders, or even of no particular
disorder at all.
Syringomyelia can be very deceptive because some symptoms (which may
include paw licking, head shaking, head rubbing, circular walking, fly
biting, and reluctance to defecate) are common behaviors for many unaffected
dogs. One distinction is that dogs suffering from SM engage in these
patterns excessively and seemingly compulsively. So, other causes of the
dog's symptoms need to be considered and should be ruled out before
concluding that SM is the cause. For example, if a syrinx develops in a
lower area of the spine, such as the lumbar region, the dog may scoot
excessively, even to the extent of rubbing the anal area raw. However,
scooting is a common symptom of other disorders, or even of no particular
disorder at all.
(Photo is of a cavalier writhing in pain from CM/SM. Photo is from Pedigree Dogs Exposed.)
In a 2009 study of 64 cavaliers affected with CM/SM, Drs. Sofia Cerda-Gonzalez, Natasha J. Olby and others classified clinical signs of pain from grade 0 to grade 5, by which the dogs displayed symptoms of neck scratching, head scratching, neck pain upon neurolgoical examination, as well as ataxia and paresis detected upon examination. See table below:
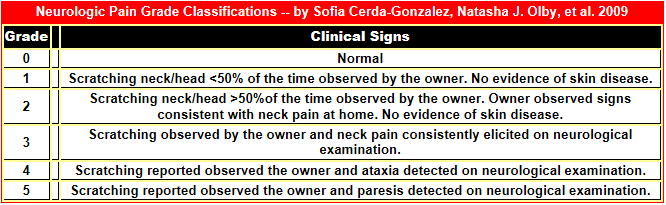
In a September 2017 study of CM and SM in Chihuahuas, the researchers used a questionnaire for the dogs' owners to complete. The questions for dogs included a grading system for the description of the presence frequency, and severity of CM/SM-related clinical signs, such as:
1.persistent scratching episodes of the ears or shoulders with or without skin contact
2.persistent scratching episodes of the cranial thoracic spine with or without skin contact
3.facial rubbing
4.spinal hyperesthesia
5.vocalization
6.gait incoordination
7.weakness
These were graded from 1 (occurring <2 times a week) to 5 (occurring several times a day). A percentage from the maximum points (7x5 = 35 points) was calculated for each patient.
In a 2010 Canadian study, researchers found a significant linear correlation between the severity of neurologic dysfunction and size of the syrinx, with a larger syrinx being associated with more severe neurologic signs.
In an April 2012 study, Geoffrey Skerritt and Dr. Luca Motta observed that the level of neurological pain a dog experiences can only be based upon subjective evaluations of the dog's behaviors, which includes the dog's owner's subjective observations.
In order to evaluate changes in the level of a dog's discomfort as objectively as possible, to determine whether their surgical procedure on the dog was successful (see "syringosubarachnoid shunt" below) these neurosurgeons devised a Pain Score Scheme (see table below) for the dog's owners to measure pain which the dog experiences from CM and SM, particularly following surgery. Their pain score scheme was created on the basis of different neurological grade classifications previously suggested by other researchers, including the Cerda-Gonzalez / Olby 2009 study above. Mr. Skerritt and Dr. Motta evaluated their patients' histories and found some specific information that could be used to create an objective pain score (i.e., frequency of scratching episodes and site of scratching, screaming episodes). However, because of the inherent subjectivity of relying upon reports from the dog's owners, they concluded that the design of a more robust scoring system together with prospective studies was warranted.
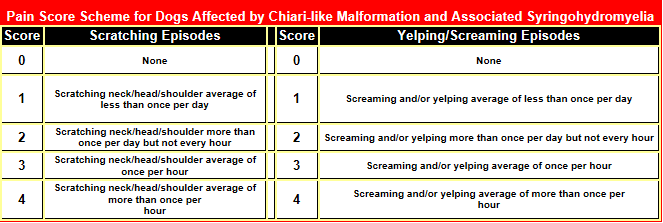
In a 2012 study, UK researchers of cavaliers with neuropathic pain report on the results of far more extensive questionnaires (using a 5-point scale), completed by the owners of 122 CM/SM-affected CKCSs. They found that owners who noticed evidence of neuropathic pain in their dogs also found the dogs to have increased fear-related behaviors (such as acting more fearfully when approached by strangers, or when in unfamiliar situations, or when sudden loud noises occurred, such as thunderstorms). These dogs also were more clingy to their owners and appeared to be more fearful when left alone. They also showed decreased willingness to exercise, and problems in settling, including sleep disturbances. Not surprisingly, the study also showed that owners found that their affected dogs had reduced quality of life.
In an effort to pinpoint the locus of the pain caused by SM, in an August 2012 study, researchers compared the expression of two pain-related neuropeptides* in the spinal cord dorsal horn of normal dogs with the peptides' expression in cavaliers with and without clinical signs of syringomyelia. They discovered that there was a decrease in expression of both peptides in CKCSs with symptomatic syringomyelia.
*Peptides are molecules formed by joining from two to about fifty amino acids. The two neuropeptides in this study were substance P and calcitonin.
In
an
April 2013
study of 26 cavaliers (11 dogs without clinical signs of pain; 6 dogs
with pain and symmetrical syrinxes; 9 dogs with pain and asymmetrical
syrinxes), German researchers
found "an association" between pain and SM
asymmetry, and they found "a strong association" between pain and dorsal
horn involvement of SM. CKCSs with clinical signs of pain showed either
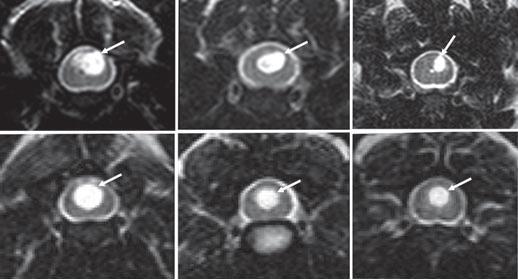 asymmetrical
syrinxes or involvement of the dorsal horn gray matter. They also found that
cavaliers with clinical signs of pain showed a presence of interleukin-6 (a
key component of the nervous system's response to injury) and substance P (a
neurokinin that regulates the immune functions of spinal glial cells) in
their cerebrospinal fluid (CSF). The researchers conclude that the release
of interleukin-6 and substance P is a factor in the development of
persistent pain in cavaliers with SM. They suggest that this information
could offer new diagnostic and treatment options for CKCSs with SM. (In
the study's photo above, the top three syrinxes are asymmetrical; the bottom
three are symmetrical.)
asymmetrical
syrinxes or involvement of the dorsal horn gray matter. They also found that
cavaliers with clinical signs of pain showed a presence of interleukin-6 (a
key component of the nervous system's response to injury) and substance P (a
neurokinin that regulates the immune functions of spinal glial cells) in
their cerebrospinal fluid (CSF). The researchers conclude that the release
of interleukin-6 and substance P is a factor in the development of
persistent pain in cavaliers with SM. They suggest that this information
could offer new diagnostic and treatment options for CKCSs with SM. (In
the study's photo above, the top three syrinxes are asymmetrical; the bottom
three are symmetrical.)
 In
a
May 2016 article, UK researchers tested the electronic von Frey
aesthesiometer (eVF) (at right) on twelve cavaliers to determine if
they could quantify the dogs' cervical skin sensitivity. They decided that the
number of dogs was insufficient to reach any conclusions, and they announced
plans to study a larger group of CKCSs and divide them by CM/SM status. Their
aim is to establish a protocol to quantify neck pain in cavaliers with
neuropathic pain.
In
a
May 2016 article, UK researchers tested the electronic von Frey
aesthesiometer (eVF) (at right) on twelve cavaliers to determine if
they could quantify the dogs' cervical skin sensitivity. They decided that the
number of dogs was insufficient to reach any conclusions, and they announced
plans to study a larger group of CKCSs and divide them by CM/SM status. Their
aim is to establish a protocol to quantify neck pain in cavaliers with
neuropathic pain.
In a November 2016 abstract, UK researchers (H. Williams, S. Sanchis, H. A. Volk, L. Pelligand, J. Murrell, N. Granger) tested 70 cavalier King Charles spaniels for skin sensitivity using the eVF. The dogs were categorized in three classes: (i) 37 dogs had syringomyelia and clinical signs (syringomyelia-symptomatic - SM-S); (ii) 15 dogs had syringomyelia without clinical signs (syringomyelia-asymptomatic - SM-A); and (iii) 18 dogs had no syringomyelia (syringomyelia-free - SM-F). The researchers found that eVF assessment of skin sensitivity does not differ significantly by syringomyelia status.
In a July 2019 article, a team of UK neurology researchers studied the records of 130 cavalier King Charles spaniels diagnosed with Chiari-like malformation (CM) and some also with syringomyelia (SM) to determine which symptoms (clinical and behavioral signs) related to CM and to SM and to syrinx diameter, in order to use the data in future studies of diagnosis, treatment, and genetics of CM/SM. Dogs were grouped based upon whether they had no SM (Group 1) up to having a syrinx greater than 4 mm (Group 4).
Signs found TO BE RELATED to syrinx presence or size:
• Phantom scratching -- 67% of Group 4 dogs and none in other groups
• Scratching or rubbing of the head or ears -- 28% but less common in Group 4 dogs
• Scoliosis -- 27% of Group 4 dogs and none in other groups
• Postural defects -- 15% of Group 4 dogs and none in other groups
• Weakness -- 39% of Group 4 dogs and none in other groups
The researchers stated that their findings suggest that "phantom scratching is highly unlikely with small syrinxes." They also found that among the dogs in their study, PSOM (primary secretory otitis media) is common in symptomatic CM-affected dogs and in SM-affected dogs, thereby raising the possibility of confusing the cause of some signs and behaviors, since PSOM-affected dogs tend to engage in ear-rubbing. They concluded:
"The study further suggests that SM-specific signs are phantom scratching, scoliosis, and sensory and motor signs that can be related to spinal cord damage by the syrinx and are associated with large syringes (transverse width >4 mm). Non-SM-specific signs include vocalization (described as without obvious trigger, when shifting position when recumbent and when being lifted under the sternum to a height), spinal pain, head and ear rubbing or scratching, aversion to touch, refusal or difficulty jumping or doing stairs, exercise intolerance/reduced activity, sleep disruption, or behavioral change described as becoming more anxious, timid, aggressive, or withdrawn. These non-SM-specific signs could reflect CM-P [CM associated pain]."
The implication from this study is, therefore, that the major signs of pain expressed by CM/SM cavaliers definitely are not due to having a syrinx and probably are due to having Chiari-like malformation, but the authors cannot definitely attribute the painful expressions solely to CM.
In a January 2025 YouTube video, Dr. Clare Rusbridge observed that as CM-affected cavaliers grow older, their brains shrink, and as a consequence, the severity of fhe CM -- and thus the resulting pain -- may reduce.
RETURN TO TOP
Changes in barometric pressure
Some owners of CM/SM-affected dogs have reported anecdotally that changes in barometric pressure due to weather conditions appear to affect the levels of pain in their dogs. Scientifically-structured studies on this topic have been limited to owners' answers to researchers' questionnaires using grading scores, such as a scale of 1 to 10, and therefore are not very objectively reliable. Only two such reports, discussed here below, have been found, and in both of them, the conclusion is that no evidence supports the hypothesis that barometric changes affect pain level variations.
In an April 2015 report, UK researchers compared daily barometric pressure changes with the comfort levels (reported by dogs' owners in answers to questlonnaires) of 22 cavaliers affected by CM/SM for 3 months. They found no evidence supporting an association between barometric pressure and the degree of pain symptoms displayed by CKCS with CM/SM. They concluded:
"Currently, there is no evidence supporting an association between barometric pressure and the degree of discomfort experienced by CKCS with CM/SM. This appears contrary to the experience of some individuals affected by these conditions. A more objective assessment of a larger population of dogs is required to determine whether or not barometric pressure has an influence on the comfort of some or all CKCS with CM/SM."
In a May 2015 master's thesis, a Netherland's researcher reviewed the answers to questionaires by the owners of 848 cavaliers, 45 of which (19.3%) had clinical signs of CM/SM. The questions included changes in pain symptoms related to different weather conditions, including cold/ hot temperatures and high/low barometric pressures. The researcher stated at the outset that some dogs appear to show more extreme symptoms during cold weather or low barometric pressure. However, the author reported:
"In this research there was no significant relationship between the different weather influences and the sensitivity of the CKCSs. This could be because owners are not really aware of the barometric pressure and they do not link the grade of the symptoms with the barometric pressure. ... To really evaluate the effects of barometric pressure and temperature on neuropathic pain in CKCS it would be more reliable to do research in a climate controlled room and stimulate the dogs in different ways to observe if they react more painful during the different weather conditions."
RETURN TO TOP
Other disorders with similar symptoms
Several of these signs are so common that they may be for other reasons and unrelated to CM or SM at all. For example, prior studies have shown that approximately 25% of dogs that display clinical signs of SM are not found to have a syrinx on MRI scans. See, e.g., this December 2011 article.
Another disorder common to cavaliers and with symptoms similar to SM is Primary Secretory Otitis Media (PSOM), which is a highly viscous mucus plug which fills the middle ear and causes the tympanic membrane to bulge. Because the pain and other sensations in the head and neck areas, resulting from PSOM, are so similar to symptoms due to SM, the possibility that the cavalier has PSOM and not SM should be determined before diagnosing SM.
In a brief July 2009 article, UK researchers Dr. Richard J Piercy and Gemma Walmsley disclosed that they had identified a genetic form of muscular dystrophy in the cavalier, with symptoms (weakness and exercise intolerance) similar to some of those of SM. However, these other symptoms of this muscular dystrophy may clearly distinguish it from SM: muscle atrophy, difficulty swallowing, and an enlarged tongue. Also, the researchers have found that only males are affected by this form of muscular dystrophy, and the females are only carriers of the mutation.
Dr. Curtis Dewey has reported that in the course of his examination of MRIs of cavaliers with Chiari-like malformation, he also has discovered cerebellar infarcts (strokes). He has written that CKCSs appear to be pre-disposed to infarcts due to the presence of CM and that the existence of both CM and infarcts "is common in the CKCS." See Cerebellar Infarcts for details.
RETURN TO TOP
Symptoms not due to CM/SM
Scratching the belly or chewing the paws are not signs of CM or SM. If a dog engages in phantom scratching but does not have a wide syrinx extending to the superficial dorsal horn (SDH) in the C3-C6 spinal segments, then that dog most likely is not displaying a symptom of SM.
Seizures are not caused by CM or SM. Progressive paralysis of the hind legs is not caused by CM or SM. CM/SM-affected dogs may display some weakness in their hind legs, and they may stumble when they walk, but they keep on walking nonetheless. So, paralysis of the hind legs indicates, more likely, intervertebral disc disease or degenerative myelopathy.
Behaviors and physical signs customarily attributed to epilepsy, fly catching, facial nerve paralysis, vestibular (balance) disease, intervertebral disc disease, and degenerative myelopathy (severe and progressive hind limb weakness) are either not caused by CM or SM or are highly unlikely to be associated with either CM or SM. These disorders do not result in structural changes on MRI scans.
RETURN TO TOP
Diagnosis
- Chiari Check Questionnaire
- CHASE Questionnaire
- Magnetic resonance imaging (MRI)
- Computed tomography (CT)
- Thermography (Medical infrared thermal imaging)
- Ultrasound
- Brainstem auditory evoked response (BAER)
- Physical examination
- Machine learning
- Severity of symptoms
- Biomarkers
- Gabapentin trial
Chiari Check Questionnaire
Chiari Check is a free, on-line questionnaire-based diagnostic tool which estimates the likelihood of Chiari-associated pain (CM-P) and syringomyelia-associated phantom scratching, based upon owner-reported answers to the questionnaire. This tool is intended to support early decision-making, in advance of magnetic resonance imaging (MRI), and improve access to diagnosis for CM/SM-affected dogs, worldwide. Accurate dagnosis requires magnetic resonance imaging (MRI) scanning, but limited access and high costs often delay treatment. Watch this explanatory video on YouTube at this link.
In this September 2025 poster presented at the 2025 European College of Veterinary Neurology (ECVN) Symposium, the results of submissions of 1,091 dogs of 62 breeds and cross-breeds, 80% of which were cavalier King Charles spaniels, are described.
RETURN TO TOP
CHASE Questionnaire
In a January 2026 article, researchers at North Carolina State University and Ohio State University devised a questionnaire for owners of dogs diagnosed with Chiari-like malformation and syringomyelia (CM/SM) to assess their dogs' responses to pain treatment with pregabalin and placebo during periods of 2 weeks each. There were 2 groups of dogs, a healthy group of 20, including 2 cavalier King Charles spaniels, and a CM/SM-affected group of 30 cavaliers. Each week of treatment, the owners completed the CHASE (Chiari-like malformation and syringomyelia evaluation) questionnaire to assess their dogs' response to treatment. The CHASE questionnaire consists of 5 categories -- Scratching (displaying visible signs of irritation), Anxious (displaying nervousness or worry), Sensitive (easily upset or hurt), Uncomfortable, and Restless -- and the main question is "Please think about how your dog has behaved over the past week and use the scale to tell us how well the phrases describe your dog or what he/she is doing." They concluded:
"Our results support that the CHASE questionnaire is capable of successfully capturing behaviors that are believed to be a consequence of neuropathic pain and itch in dogs with CM/SM. This conclusion is strongly supported by the responsiveness of the component questions and overall score to the clinical signs of the condition. The final CHASE questionnaire was simple to administer remotely, owners answered the questions consistently, and it discriminated between healthy and affected dogs. In addition, it detected appropriate changes in clinical signs in canine CM/SM patients receiving a treatment of known efficacy in a double-blinded, randomized controlled trial. Robust repeatability also was observed, evidenced by the lack of significant difference in CHASE scores at both timepoints in the healthy cohort and between screening and baseline CHASE scores in the CM/SM cohort."
RETURN TO TOP
Magnetic resonance imaging (MRI)
 The
most accurate way of diagnosing the Chiari-like malformation and
syringomyelia is
said to be through the use of
magnetic resonance imaging (MRI) scanning.
The MRI allows the veterinary neurologist or neurosurgeon to study the
skull and spine for the presence of any abnormality which might
obstruct the flow of the cerebrospinal fluid. When examined by MRI,
the syringomyelia appears as a tubular cavity of fluid, called a syrinx,
within the spinal cord. In severe cases, the syrinx is so wide that only a
thin rim of the spinal cord is visible. An MRI scan of a dog without
any syrinxes at all still may show that the dog has Chiari-like
malformation.
The
most accurate way of diagnosing the Chiari-like malformation and
syringomyelia is
said to be through the use of
magnetic resonance imaging (MRI) scanning.
The MRI allows the veterinary neurologist or neurosurgeon to study the
skull and spine for the presence of any abnormality which might
obstruct the flow of the cerebrospinal fluid. When examined by MRI,
the syringomyelia appears as a tubular cavity of fluid, called a syrinx,
within the spinal cord. In severe cases, the syrinx is so wide that only a
thin rim of the spinal cord is visible. An MRI scan of a dog without
any syrinxes at all still may show that the dog has Chiari-like
malformation.
Clinic charges for MRI examinations of canines have been known to vary from $400.00 to over $2,000.00.* Accurate MRI results require that usually the dog be anesthetized. In view of the high cost of MRI scans, the examing veterinary specialist usually will attempt to rule out other causes of the symptoms first. Veterinarians who perform MRIs of should consider following this MRI Screening Protocol devised by Dr. Rusbridge.
*See our list of low-cost MRI clinics here.
While MRI is considered "the gold standard" in diagnosing both CM and SM, as well as the degree of each, MRI is not without error. MRI is limited to providing images of the structure of the brain and spinal column. It requires the examiner to make certain viewing decisions, such as the angle for transverse images, and if that angle is not perpendicular to the dog's spinal cord, the images may artificially enlarge the central canal and result in an incorrect diagnosis. Also, the abilities and experience levels of the examiners have been shown in studies to play an important role in the accuracy of their analyses. Radiology diplomates with decades of experience are much better equipped to accureately diagnose the disorders and grade their degrees than are less experienced examiners. See this April 2020 article for a discussion of the comparisons in abilities of MRI examiners regarding cavaliers with or without CM and SM.
MRI scans cannot confirm that pain is due to either CM or SM. If a dog diagnosed with CM/SM displays indications of pain, the neurologist must eliminate other possible causes of that pain.
The MRI scan of a cavalier below at the right shows the occipital malformation, with the cerebellum being squeezed out of the occipital bone and into the area of the foramen magnum (red-outlined area). It also shows pockets of white cerebrospinal fluid in the spinal cord (yellow-outlined area). See Karen Kennedy's Basic Canine NeuroAnatomy and MRI Imaging Planes, for further information about MRI scans.
In a
2011 study conducted by Drs. Rusbridge and Knowler,
in a sample of seventy "unaffected" cavaliers from Europe and North America, which were
MRI-scanned only for breeding purposes, 70% of them had syringomyelia, 17%
were "at risk", meaning were young dogs with Chiari-like malformation but no
syringomyelia yet, and only 13% were "clear" of both the malformation and
SM. In February 2010, Dr. Georgina Child, board certified veterinary neurologist in
Australia, reported that of 60 asymptomatic cavaliers scanned as potential
breeding stock, 50% had SM syrinxes.
In MRI studies of 49 cavaliers, reported in 2011 in the Veterinary Journal, Dr. Rusbridge and others found that "Syrinx formation was present in the C1-C4 region and in other parts of the spinal cord. The maximal dorsoventral syrinx size can occur in any region of the spinal cord." Seventy-six per cent of CKCS with a a cranial cervical syrinx also had a syrinx in more caudal spinal cord regions. Therefore, so-called "mini-MRI-scans" of only the cervical region, such as those scans for breeding protocol purposes, may not necessarily locate all syrinx which an SM-affected cavalier may have.
Dr. Curtis Dewey has reported that in the course of his examination of MRIs of cavaliers with Chiari-like malformation, he also has discovered cerebellar infarcts (strokes). He has written that CKCSs appear to be pre-disposed to infarcts due to the presence of CM and that the existence of both CM and infarcts "is common in the CKCS." See Cerebellar Infarcts for details.
Also, benign, small syrinxes are a common incidental finding on MRI
examinations. Therefore, clinical
correlation is important in order
to determine if the syrinx is associated with the Chiari-like
malformation and/or symptoms.
The following MRI photographs, and their descriptive text, are courtesy of Dr. Clare Rusbridge and Dr. Penny Knowler of Stone Lion Veterinary Centre:
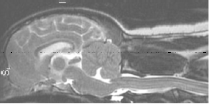 Left: This image shows mild Chiari-like malformation - the cerebellum is
very slightly indented, the kinking of the medulla is normal for a toy breed
and there is displacement of the cerebellum into and just out of the foramen
magnum. The ventricular system is slightly dilated.
Left: This image shows mild Chiari-like malformation - the cerebellum is
very slightly indented, the kinking of the medulla is normal for a toy breed
and there is displacement of the cerebellum into and just out of the foramen
magnum. The ventricular system is slightly dilated.
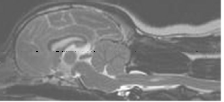 Right: Although the cerebellum is not coming through the foramen magnum,
this dog has a greater degree of Chiari-like malformation than the first
dog. The cerebellum is indented, and the medulla is kinked. The central
canal is dilated above the first disc space - this is the first sign of
syringomyelia developing. There is also mild ventricular dilatation.
Right: Although the cerebellum is not coming through the foramen magnum,
this dog has a greater degree of Chiari-like malformation than the first
dog. The cerebellum is indented, and the medulla is kinked. The central
canal is dilated above the first disc space - this is the first sign of
syringomyelia developing. There is also mild ventricular dilatation.
 Left: This dog has descent of the cerebellum towards the foramen magnum and the
cerebellum is indented. The medulla is normal for a toy breed; there is mild
ventricular dilatation and a small syrinx/central canal dilatation in the
upper cervical spinal cord.
Left: This dog has descent of the cerebellum towards the foramen magnum and the
cerebellum is indented. The medulla is normal for a toy breed; there is mild
ventricular dilatation and a small syrinx/central canal dilatation in the
upper cervical spinal cord.
For more MRI views of cavaliers with syringomyelia or the Chiari-like malformation, see Karen Kennedy's* Understanding Canine Chiari Malformation and Syrningomyelia and Related Links below.
*Karen Kennedy, RTMR, MappSc, is a magnetic resonance imaging specialist with The London Health Sciences Centre, London, Ontario, Canada.
*Karen Kennedy, RTMR, MappSc, is a magnetic resonance imaging specialist with The London Health Sciences Centre, London, Ontario, Canada.
In a November 2018 article, Drs. Clare Rusbridge and Susan P. Knowler and Felicity Stringer have thoroughly summarized the current research knowledge about symptomatic Chiari-like malformation (CM) and syringomyelia (SM) in cavalier King Charles spaniels and other affected breeds. Their work includes handy charts covering the topics of skull changes, craniocervical junction and cervical changes, neuroparenchymal changes, syrinx features, MRI protocols, and interpretations of MRI scans for diagnosing CM pain and symptomatic SM. They also have comparison MRI scan views of cavaliers with varying stages of CM and SM, including Figure 8, below, comparing the MRI scans of three CKCSs.

• Dynamic susceptibility contrast perfusion MRI
Dynamic susceptibility contrast perfusion magnetic resonance imaging (DSC-PMRI) enables the quantification of the volume of blood passing through the brain tissue. In an August 2017 article, researchers studied the ventricle system of 42 cavalier King Charles spaniels -- 32 CKSCs with ventriculomegaly and 10 control CKCSs -- using DSC-PMRI.
• Diffusion tensor imaging (DTI)
Diffusion Tensor Imaging (DTI) is an advanced form of magnetic resonance imaging (MRI) that uses multiple MRI scans, performed in numerous different directions, of the same limited regional tissues of the brain or spinal cord (called voxels) to create a 3-dimensional image showing the directions in which water molecules diffuse in the tissue.
In a December 2022 article, a team of Polish veterinary researchers examined 30 cavalier King Charles spaniels, 18 of which were confirmed by MRI to have syringomyelia (SM) and 12 CKCSs without SM. Of the 18 CM/SM-affected dogs, 8 were symptomatic and 10 were not. All dogs underwent diffusion tensor imaging (DTI), a MRI modality which can provide a detailed assessment of the intrinsic spinal tracts and a better understanding of how tissue damage causes clinical deficiencies. It is a non-invasive MRI technique which is more sensitive to microstructural changes than conventional MR images and is able to show abnormalities within the spinal cord which are not visible on standard structural MR images. It measures microstructural characteristics of water diffusion within the nervous tissues. They report finding a difference in two DTI parameters: (1) fractional anisotropy (FA) and (2) apparent diffusion coefficient (ADC) between nonsymptomatic and symptomatic cavaliers. They concluded that the use of DTI imaging in the MRI evaluation of CM/SM dogs (and humans) may be useful in devising a protocol for an objective assessment of the spinal cord and to understand what processes lie at the basis of many diseases, the diagnosis of which is currently difficult.
RETURN TO TOP
Computed tomography (CT)
 Computed tomography (CT) is an imaging method using digital geometry
processing to generate a three-dimensional image of the inside of an object
from a large series of two-dimensional x-ray images taken around a single
axis of rotation. Researchers have been studying the value of CT scans to
detect Chiari-like malformations and syrinxes in cavaliers and comparing the
results with MRIs and other resources. In a very preliminary
2008 French study, researchers CT scanned sixteen CKCS to measure the
size of their caudal fossas and to determine standard computed tomography
dimensions of the caudal fossa.
Computed tomography (CT) is an imaging method using digital geometry
processing to generate a three-dimensional image of the inside of an object
from a large series of two-dimensional x-ray images taken around a single
axis of rotation. Researchers have been studying the value of CT scans to
detect Chiari-like malformations and syrinxes in cavaliers and comparing the
results with MRIs and other resources. In a very preliminary
2008 French study, researchers CT scanned sixteen CKCS to measure the
size of their caudal fossas and to determine standard computed tomography
dimensions of the caudal fossa.
Dr. Dominic J. Marino of Long Island Veterinary Specialists (LIVS) reported in October 2007 that evaluation of the entire skull shape and size utilizing Spiral CT technology with 3D reconstruction is currently underway to identify additional mechanisms of syrinx formation. He wrote that CT scanning may enable surgeons to focus on correcting the flow of CSF as the malformation affects its normal passage around the brain and spinal cord and leads to the syrinx formation known as syringomyelia.
In a 2013 study of nine cavaliers with neurological disorders, a team of Ghent University (Belgium) veterinary radiologists compared the dogs' MRIs and CTs and concluded:
"The statistical analysis suggested that both techniques are useful for detecting CH [cerebellar herniation]. However because the bias was significantly different from zero, one of the methods consistently led to the determination of longer or shorter HL [cerebellar herniation length] than the other method. For most comparisons, the HL was on average longer on CT. MRI provides greater soft tissue detail with no beam-hardening artifacts, which may improve the delineation of the cerebellum. Because HL does affect a diagnosis of CM, so CT can be used as a primary diagnostic tool for diagnosing CM in CKSs when MRI is not available."
In a November 2014 study of 15 cavalier King Charles spaniels by a team of Belgian researchers, they compared computed tomography (CT) scans with MRI scans and analyzing them statistically, they found "no significant difference between the different observers and techniques for the detection of CH [cerebellar herniation] and measurement of CHL [cerebellar herniation length]." However, they found, "Overall, the CHL was longer on the CT images." They concluded:
"Both techniques are useful for detecting CH and measuring CHL. Because CHL does not have a known direct impact on the clinical presentation of CM, CT can be used as a diagnostic tool in a routine clinical practice for CM in CKCS when MRI is not available. We emphasize that MRI is the standard screening technique in CKCS for breeding purposes to detect the presence of CM and SM and, at the current time, CT cannot replace MRI."
In an August 2015 report, a team of Belgium researchers compared computed tomography (CT) and magnetic resonance (MR) scans of 32 dogs, including 12 cavalier King Charles spaniels. They found that low-field MR and multislice CT imaging provided comparable information regarding the presence of SM, and that CT can be used as a diagnostic tool for SM when MRI is not available. They conclude, however, that "CT cannot replace MRI as the standard screening technique for the detection of SM in Cavalier King Charles Spaniel for breeding purposes."
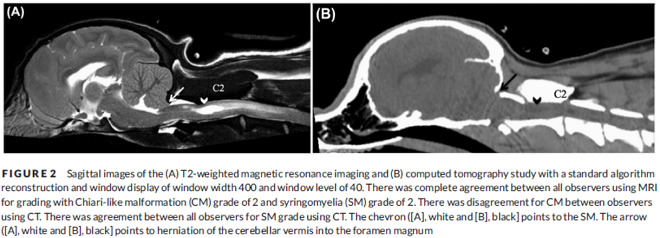
In an April 2020 article, Ohio State researchers compared MRI with CT for grading CM and SM in 30 cavaliers The study also included three classes of observers with different levels of experience -- two American college of veterinary radiology diplomates (DACVR) and two second-year veterinary radiology residents and two small animal veterinary interns. They point out the reason for the study is that, "Computed tomography is financially cheaper, shorter in acquisition time, more accessible to general practitioners, and requires less anesthetic drugs/time." (See Figure 2, above.) The results of the study support the hypothesis that the overall agreement and the agreement between observer groups of similar experience levels is higher using MRI compared to CT. As expected, the accuracy for diagnosis by experienced radiologists of cerebellar herniation and SM using MRI was higher than CT. However, CT had a higher accuracy among the second-year radiology residents and veterinary interns for identifying cerebellar herniation, which countered the investigators' hypothesis that MRI is a better tool for CM evaluation. They concluded:
"Computed tomography does not replace MRI for the diagnosis and classification of CM and SM in CKCS. The accuracy and agreement for identifying cerebellar herniation and SM in experienced observers was higher utilizing high-field MRI compared to multislice CT. Breeding programs for CKCS should continue to utilize MRI for antemortem diagnosis of CM and SM to help improve the genetic pool and minimize the risks for neuropathic pain brought about by CM and SM. Individuals with greater diagnostic imaging experience (DACVR) resulted in better agreement for both MRI and CT and higher sensitivity, specificity, and accuracy for cerebellar herniation and SM. Magnetic resonance imaging interpreted by experienced observers should remain the standard tool for CM and SM screening programs."
Additionally, CT reportedly cannot replace MRI for breeders' screening purposes, because CT cannot detect presyrinx and cannot detect fluid in the syrinx.
RETURN TO TOP
Thermography (medical infrared thermal imaging)
 Medical infrared
thermal imaging (MITI)* is a non-invasive imaging technique which records thermal
patterns. It provides information about the function of the sympathetic
nervous system. Thermal imaging has drawn the interest of veterinary
researchers as a potential screening test for CM in dogs due to its ability
to image dogs without sedation. In a preliminary 2007 study at Long Island
Veterinary Specialists (LIVS), Dr. Dominic J. Marino's team
found that cavaliers with CM had "cooler" thermographic patterns when
compared with a dog with a normal caudal fossa. Dr. Marino reported in
October 2007 that, "based on these very preliminary findings, thermography
may be a viable imaging modality to use as a screening tool to detect CLM in
dogs."
Medical infrared
thermal imaging (MITI)* is a non-invasive imaging technique which records thermal
patterns. It provides information about the function of the sympathetic
nervous system. Thermal imaging has drawn the interest of veterinary
researchers as a potential screening test for CM in dogs due to its ability
to image dogs without sedation. In a preliminary 2007 study at Long Island
Veterinary Specialists (LIVS), Dr. Dominic J. Marino's team
found that cavaliers with CM had "cooler" thermographic patterns when
compared with a dog with a normal caudal fossa. Dr. Marino reported in
October 2007 that, "based on these very preliminary findings, thermography
may be a viable imaging modality to use as a screening tool to detect CLM in
dogs."
*Medical infrared thermal imaging (MITI) is also known as medical infrared imaging (MII) and as infrared thermography (IRT).
In a June 2011 study of 105 cavalier King Charles spaniels, Drs. Marino and Catherine Loughin found that MII was up to 97.3% accurate in identifying dogs with CLM. They concluded, "Based on these preliminary findings, MII may be a viable screening tool to detect CLM in dogs." In a March 2020 article, the same team of US neurological researchers studied medical infrared thermal images (MITI) of 93 cavalier King Charles spaniels to determine if MITI could detect syrinxes in cavaliers diagnosed by MRI as having syringomyelia (SM). All 93 dogs had been examined by MRI and found to have Chiari-like malformation. Of those, 48 were diagnosed with SM and 45 had no evidence of SM. The authors tested MITI devices using a variety of texture distances, finding that a distance of 6 produced the best results -- 69.9% accuracy in detecting syrinxes known by MRI to be present. They concluded:
"This study revealed that MITI is a successful screening test for the presence of SM in CKCS with CLM. Compared to other imaging modalities, MITI is a quick, inexpensive modality that does not require sedation nor anesthesia, and eliminates radiation exposure (to patient & staff). MITI does not provide insight to syrinx location or severity nor should it be used as a sole diagnostic modality. While veterinary thermographic imaging continues to improve as advances in technology occur, MRI will remain the gold standard for definitive diagnosis and staging of Chiari-like malformation and syringomyelia."
Figure 1: Thermograms of CKCS: Left image is CKCS without SM; right image
is CKCS with SM.
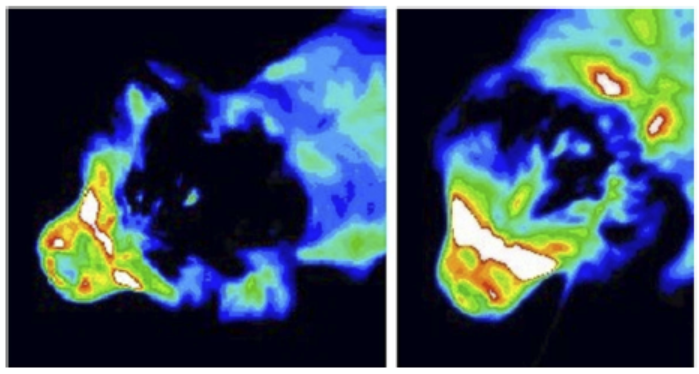
Beginning in October 2012, a UK clinic, Veterinary Thermal Imaging Limited, has been studying thermal imaging to detect of dogs to detect Chiari-like malformation and syringomyelia. The procedure used to explore the use of thermal imaging for the screening of CLM is non-invasive. Thermal images are taken of the dog's head and neck. The images can be taken without the need for sedation, and in the dog's home. The thermographer then examines these images, along with MRI results, to see if a correlation can be seen between skull and neck structures in the affected animals. Veterinary Thermal Imaging Limited is located on Hale House Lane, Churt, Surrey, GU10 2JG. For more information, contact Stephanie Godfrey at Stephanie.godfrey@vtiuk.com, telephone 0844 544 3314, website www.veterinary-thermal-imaging.com
RETURN TO TOP
Ultrasound
 In 2005, Drs.
Dominik Faissler and
John (Jay)
McDonnell, board certified veterinary neurologists at the
Cummings School of Veterinary Medicine at Tufts University in Massachusetts,
researched the use of ultrasonography to diagnose syringohydromyelia in
dogs. In a 2005 interim report, they stated, "This preliminary study
indicates that cervical spinal cord ultrasound can be useful as a diagnostic
aid for CM. It cannot rule out a diagnosis of CM, however no false positives
were found. To investigate the sensitivity and specificity of this imaging
modality blinded U/S examination of large numbers of dogs after MRI
evaluation is planned."
In 2005, Drs.
Dominik Faissler and
John (Jay)
McDonnell, board certified veterinary neurologists at the
Cummings School of Veterinary Medicine at Tufts University in Massachusetts,
researched the use of ultrasonography to diagnose syringohydromyelia in
dogs. In a 2005 interim report, they stated, "This preliminary study
indicates that cervical spinal cord ultrasound can be useful as a diagnostic
aid for CM. It cannot rule out a diagnosis of CM, however no false positives
were found. To investigate the sensitivity and specificity of this imaging
modality blinded U/S examination of large numbers of dogs after MRI
evaluation is planned."
In the same 2008 French study reported under computed tomography above, one dog's syrinx was identified by ultrasound. The researchers found that ultrasonography probably has too low a sensitivity for reliable diagnosis of Chiari-like malformation/syringomyelia.
In an August 2008 report by German researchers using ultrasound as a comparative imaging technique to MRIs, they compared 10 normal brachycephalic dogs with 25 cavaliers known to have Chiari-like malformation. They found that "Cerebellar displacement into the foramen magnum was clearly identified sonographically; however, syringohydromyelia was not discernable due to bone overlay."
RETURN TO TOP
Brainstem auditory evoked response (BAER)
 A supplemental diagnostic screening tool used by at least two veterinary
neurologists, Dr. Curtis W. Dewey and
Dr.
Georgina Barone, and by
Dr. Dominic J. Marino is
the BAER (for Brainstem Auditory Evoked Response) test. The BAER test
measures the timing of electrical waves from the brainstem in response to
clicks in the ear. Dr. Dewey reported that, assuming the dog is not deaf,
the detected brain waves can be used to assess the integrity of the brain
stem, since CM involves some degree of brain stem compression.
A supplemental diagnostic screening tool used by at least two veterinary
neurologists, Dr. Curtis W. Dewey and
Dr.
Georgina Barone, and by
Dr. Dominic J. Marino is
the BAER (for Brainstem Auditory Evoked Response) test. The BAER test
measures the timing of electrical waves from the brainstem in response to
clicks in the ear. Dr. Dewey reported that, assuming the dog is not deaf,
the detected brain waves can be used to assess the integrity of the brain
stem, since CM involves some degree of brain stem compression.
As of October 2007, Dr. Marino reported that "38 Cavalier King Charles Spaniels had been evaluated thus far. One dog had a normal MRI, BAER, and thermographic evaluation. Twenty-three dogs without clinical signs had abnormal MRI findings with 16 of the 23 dogs (69.6%) also having abnormalities with BAER testing. Fourteen dogs with clinical signs had abnormal MRI findings and 13 of the 14 dogs (92.8%) also had abnormal BAER tests. BAER testing may play a more useful role in screening 'clinical' dogs rather than dogs without clinical signs.
In a 2010 report, a group of Canadian neurologists tested fifty cavaliers to evaluate the validity of BAER as well as transcranial magnetic motor evoked potentials (TMMEP), somatosensory evoked potentials (SSEP), and spinal evoked potentials (SEP), compared to MRIs. The researchers found: "TMMEP, SSEP, SEP and BAER do not appear to be valuable tests in detecting functional abnormalities of the motor and sensory pathways throughout the central nervous system of CKCS dogs with and without neurological signs secondary to SM diagnosed by MRI."
RETURN TO TOP
Physical examination
In a 2014 study of 133 cavaliers, researchers examined their skulls to determine if skull measurements could predict the presence or development of syringomyelia (SM), They found that as the dog's cranium is shortened and broadened, the risk of developing SM increases. They stated:
"The study found two aspects of conformation to be associated with the development of SM in the CKCS: the cephalic index and the distribution of cranium across the length of the head. It was found that a higher cephalic index and, separately, a lower percentage of the cranium distributed caudally were significantly associated with disease development."
"The cephalic index is the ratio of the width of the cranium of an organism (taken behind the cheekbones in this study) divided by its length (i.e., in the horizontal plane, or front to back). It is usually expressed as a %. It differs from craniofacial index in that it does not relate to the length of the muzzle."
"The conformational indicator of caudal cranium distribution was found to significantly, correctly classify cases as SM clear or affected at the level of three years of age, five years of age, and when comparing a sample of SM clear dogs over five years to those affected and younger than three. Cephalic index was able to significantly, correctly classify cases at the latter level. Results suggest that these indicators are irrelevant of age (after 18 months of age), gender and parity. These, therefore, represent invaluable tools in determining breeding plans in that they are not only protective against developing the condition in the first three years of life but they are protective against developing the condition at all, maintaining SM clear status beyond the age of five years."
![]() This
confirmation indicator research is not a diagnosis, but is to aid in
risk assessment to provide breeders with a tool to use with their
breeding stock. See
also this
YouTube video explaining this article.
This
confirmation indicator research is not a diagnosis, but is to aid in
risk assessment to provide breeders with a tool to use with their
breeding stock. See
also this
YouTube video explaining this article.
In a June 2018 abstract presented to the 2018 ACVIM Forum, UK researchers relied upon an innovative machine learning technique (a computerized data analytics technique using computational methods to learn information from data without relying on a predetermined equation as a model). The team obtained MRI scans of 66 cavalier King Charles spaniels (CKCSs) over the age of 4 years. Of them, 26 did not have syringomyelia (SM) and 40 had SM with a syrinx width of at least 4 mm. They performed morphometric analysis of the shape and position of the soft palate in relation to the skull base and the rostral (towards the oral or nasal region) flattening of the forebrain. In the SM dogs, the distance between the rostral (again, towards the oral or nasal region) end of the soft palate and (a) the sella turcica (a saddle-shaped depression in the body of the sphenoid bone of the human skull, where the pituitary gland sits), and (b) the foramen magnum basioccipital portion of occipital bone, was significantly shorter than the non-SM control group of dogs. The shorter distance between the brain and the frontal bone also was highly significant in SM-affected dogs. They concluded that CKCSs with SM have a flattening of the frontal portion of their skulls, when compared to non-SM cavaliers.
In a January 2019 article by a team of UK and Swedish researchers, 13 cavalier King Charles spaniels were examined by UK breed judges, using a checklist, to determine if the risk of Chiari-like malformation (CM) and syringomyelia (SM) could be identified by visual assessment of head shape. The results showed a positive correlation between the judges' evaluations and the risk of CM/SM sufficient to warrant a larger study of the breed. Figure 5 (below) shows the most extreme range of checklist scores. The researchers concluded:
"This prospective investigation demonstrated that it was possible to compare subjective evaluation of head conformation with objective measurements and revealed a significant correlation between the subjective visual evaluation of head conformation and an objective evaluation of dorsoventral doming using photographs. However, this pilot investigation demonstrated that individual adjudicators can vary in their interpretation of the CKCS breed type and also suggests that measuring the cephalic index or rostrocaudal doming alone is not a reliable indicator of brachycephaly but should be taken together with a visual evaluation and take account of other features, such as those on the checklist and the size of the dog."

In a November 2019 article, a team of UK researchers reviewed the medical records of 66 cavalier King Charles spaniels (CKCS), 40 of which had syringomyelia (SM) and the other 26 did not; 55 had Chiari-like malformation (CM) and 11 did not. The dogs were grouped by (1) control group of 11 with no Chiari-like malformation (CM-N); (2) CM pain group (CM-P) of 15 dogs; (3) clinical SM group (SM-S) of 40 dogs. SM-S dogs included those with outward symptoms of SM (variable phantom scratching, scoliosis, etc.) and a syrinx of at least 4 mm. The researchers divided their study into two sub-sets, the first examined head features related to the dogs' soft palates, and the other examined features related to their hard palates; both sub-sets also included review of the dogs' features related to forebrain flattening and olfactory bulb rotation. The olfactory bulb is a bulb of neural tissue within the dog's fore-brain. Their work included comparing the shape of the "stop" of each dog, which is the degree of the angle where the nose and skull meet, and the indentation between the eyes at that point. A "gentle stop" has the least angular shape and a "pronounced stop" has the sharpest angle.
They found (see figure 5 below):
• CM-N dogs (no CM) had the least brachycephalic head, a gentle stop with the greatest upper jaw area between the hard palate and the frontal bone, and the longest soft palate length.
• CM-P dogs (painful CM) had the least distance between the hard palate and cranium, a pronounced stop, and a displaced olfactory bulb.
• CM-S dogs (large syrinx) had the most reduced middle craial bone area and shortest distance between the connection of the hard and soft palates with the base of the cranium.
They conclude that dogs with CM-P had the shortest muzzle lengths, and that "a reduced distance between the hard palate and the frontal bone was particularly associated with CM-P." Dr. Clare Rusbridge, one of the researchers, explained:
"Dogs with clinically relevant CM/SM are more likely to have brachycephalic features of the rostral skull flattening with reduction of nasal tissue and a well-defined stop. This evidence not only enhances our understanding of the disease and 'at risk' head conformation but could also impact on the assessment of MRI and disease diagnosis. It suggests the whole skull should be analyzed and not just the hindbrain currently required in prebreeding screening. This information has implications not only for breeders and pet owners but also for the veterinary profession to raise awareness about the welfare aspects of breeding. Furthermore, an increased risk for SM and painful CM might not be confined to brachycephalic breeds but other miniaturized purebreeds and hybrids that have gained in popularity as pets."
Co-researcher Dr. Susan P. Knowler explained:
"This study suggests that the whole skull, rather than just the hindbrain, should be analysed in diagnostic tests. It also impacts on how we should interpret MRI from affected dogs and the choices we make when we breed predisposed dogs and develop breeding recommendations. ... The brachycephalic features that can be seen from outside is a head that has flattening at the front with reduction of nasal tissue and a well-defined stop."
 Ga
Ga
RETURN TO TOP
Machine learning
"Machine learning" (ML) is an artificial intelligence device using statistical algorithms and models to analyze and draw inferences from patterns of information.
Chiari Check is
a free, web-based diagnostic tool developed to aid in
assessing the likelihood of pain
 caused by Canine Chiari malformation
and syringomyelia in dogs. Chiari Check uses a Machine Learning model,
enabling veterinarians and caregivers to upload clinical signs via a
detailed questionnaire. The tool uses algorithms to predict the
likelihood of disease.
caused by Canine Chiari malformation
and syringomyelia in dogs. Chiari Check uses a Machine Learning model,
enabling veterinarians and caregivers to upload clinical signs via a
detailed questionnaire. The tool uses algorithms to predict the
likelihood of disease.
UK researchers Kristiana Ivanova, Clare Rusbridge, and Mariam Cirovic introduced Canine Check at the March 2024 BSAVA 2024 conference, They presented a poster (at right -- click to enlarge it) announcing the creation of a machine learning tool available online that can predict the likelihood of painful Chiari-like malformation (CM-P) in cavaliers and other breeds. In this project, data from 185 dogs diagnosed with CM-P and syringomyelia (SM) together with the dogs' owners answers to a series of questions about their dogs' symptoms and behaviors. The model program called Naive Bayes model ("MICE NB all combined") performed best against the evaluation metrics and test data, for both predicting CM-P and SM.
The researchers concluded that their tool supports suspected diagnosis when further diagnostics, such as MRI scans, are restricted due to their costs. They stated that:
"The collected data provides researchers better insight into common clinical indicators of CM-P and SM for diagnosis, monitoring effectivenes of treatment, and assessing quality of life."
RETURN TO TOP
Severity of symptoms
In the absence of an MRI, the severity of certain SM-related symptoms and their combinations have been found to indicate the size range of syrinxes in cavaliers. In a February 2024 article, Danish researchers investigated the symptoms of 89 cavaliers which had been diagnosed with SM by MRI at the University of Copenhagen, Denmark. They interviewed the dogs' owners, to determine the relationship between the sizes of the dogs' syrinxes and their SM-related symptoms. The severity of the dogs' CM and SM were cagtegorized by the British Veterinary Association/Kennel Club scheme of measuring the condition of the CM and sizes of the syrinxes. The dogs were distributed into three groups: (1) dogs with CM and no SM (or with a maximum transverse width < 2 mm), (2) dogs with CM and small syrinx (SM 2.00-3.99 mm) and (3) dogs with CM and large syrinx (SM >4 mm). They report finding that these clinical signs were reported significantly more frequently in dogs with large syrinxes:
• Phantom scratching
• Scratching of both sides of the neck and shoulders
• Aversion to being touched to the head, neck, or shoulders
• Worsening of clinical signe when the dog was emotionally aroused
• Preferred head posture during sleep
They found that when phantom scratching, aversion to touch, and a preferred head posture while asleep were combined, the likelihood of a large syrinx increased. They concluded that clinicians may use this information to diagnose CKCSs with a large syrinx and necessary treatment when MRI diagnosis is not available or affordable.
RETURN TO TOP
Biomarkers
Calcitonin gene related peptide (CGRP) is, obviously, a peptide. It is produced primarily by cells in the thyroid and is secreted into the nervous system, where it is stored.
In a June 2024 report to the ACVIM Forum 2024, In a study of29 cavaliers, 15 were diagnosed with CM/SM by MRI. Of those 15, 13 displayed symptosms of pain and 12 were scratching. Lumbar CSF was collected from each dog, and concentrations of CGRP from the CSF was correlated with the clinical signs of pain and itching. The investigators found that CGRP concentrations were elevated in cavaliers exhibiting pain and itching. The investigators concluded that CGRP may contribute to neuropathic pain in CM/SM-affected dogs, and that it could be a target for therapy.
RETURN TO TOP
 Gabapentin trial
Gabapentin trial
If the dog is suspected to have CM/SM but its symptoms are mild, it may not be necessary to have the dog diagnosed by MRI or any of the electronic alternatives described above. A thorough physical examination by a neurologist may be all that is needed to enable the veterinarian to prescribe medications, typically gabapentin, which may manage the suspected CM/SM. This commonly is called a respnse-to-treatment approach, and more specifically a "gabapentin trial". If the drug appears to resolve the symptoms, or at least improve them, then SM may be assumed without having to conduct further diagnosis. This may be the best course if the dog also has mitral valve disease or is elderly and the owner does not want the dog to have to endure the anesthesia necessary to conduct the MRI.
RETURN TO TOP
 Reduced Rate MRI Clinics
Reduced Rate MRI Clinics
in United States and Canada*
The names and locations of veterinary neurologists who are board certified by the American College of Veterinary Internal Medicine (ACVIM) are on our Neurologists webpage. Some MRI clinics which are offering reduced rates for partial scans of cavaliers are listed below. Be aware that prices for MRI scans at these clinics are subject to change and may not include other services which are necessary for a complete analysis of the dog's condition. Veterinarians who perform MRIs are advised to follow the MRI Screening Protocol.
(* The UK's cavalier club also offers this list of MRI clinics throughout the world.)
AUBURN, AL:
Auburn University's small animal teaching hospital is offering MRI scans for $1,010.00,
limited to cavaliers and Brussels griffons at least one year old and
asymptomatic (showing no signs of CM/SM). Appointments are available only on
Thursdays. Pre-anesthetic testing (blood work and thoracic radiographs) must
be performed by a veterinarian elsewhere within two weeks of the CM
screening appointment. The CM screening package includes:
•
Physical and neurologic examination by the neurology/neurosurgery service
• General anesthesia
• MRI of the head/neck
• Two CD
copies of the MRI images
• Breeding advice based on a previously
published grading scheme from the British Veterinary Association.
Bailey
Small Animal Teaching Hospital is located at 1220 Wire Rd., Auburn, AL
36849. Contact Dr. Amy B. Yanke (Email:
aby0003@auburn.edu) at 334-844-4690
for more information. Website:
www.vetmed.auburn.edu
LOS ANGELES, CA: Dr. Veronique Sammut, board certified veterinary neurologist, offers mini-scans for $1,220.00, which include mini MRI, IV catheter and IV fluids during anesthesia, a copy of the MRI on CD and interpretation. The scan is from the inter-thalamic adhesion to C5 or lower. It also covers axials of the tympanic bullae to evaluate for PSOM ("glue ear"). A consultation is required prior to scheduling procedure, not included in price above. Pre anesthetic work up within 3 weeks of planned anesthesia is also required, also not included in price above. Contact Lily Medrano at VCA-West Los Angeles, at 1900 S. Sepulveda Blvd., Los Angeles, CA, 90025, Tel: 310-473-2951, Email: lily.martinez@vca.com, website: https://vcahospitals.com/west-los-angeles/specialty
REDWOOD CITY, CA: AnimalScan at 410 Brewster Avenue, Redwood City, CA 94063, telephone 650-480-2001, email info@animalscan.org website animalscan.org
NAPLES, FL: Dr. Michelle Carnes, board certified veterinary neurologist at Animal Specialty Hospital of Florida, offers an MRI package for asymptomatic (non-clinical) patients at $900.00 (for three or more dogs), $1,000.00 (for two dogs), and $1,100.00 for one dog. The MRI scan is a mini-scan from interthalamic adhesion to C5. The price includes a neurologic exam/physical prior to the scan and a consultation following the scan for review of study, anesthesia, a written interpretation of the scan, and a copy of the MRI on a CD. Bloodwork is required and is not included in the price. Bloodwork should be no older than two weeks. A report from a board certified veterinary radiologist can be obtained for an additional fee. For more information, contact Eric Carnes, telephone 239-263-0480, email ecarnes@ashfl.com. Animal Specialty Hospital of Florida is located at 10130 Market Street, Suite 1, Naples, Florida 34112, telephone: 239-263-0480, fax: 239-263-0488, website: www.ashfl.com
FORT WAYNE, IN: Advanced Animal Imaging offers Cavalier breeders a $495.00 mini-scan MRI per dog, which includes a consultation, reading of the scan, and anesthesia. Pre-screening bloodwork is required prior to anesthesia and is available for $75.00 at the Indian Creek Veterinary Hospital in the same building. The clinic follows Dr. Rusbridge's SM MRI screening protocol. Contact the clinic at telephone 260-434-1555 to make appointments. Advanced Animal Imaging is located at 5902 Homestead Road, Fort Wayne, IN 46814, and its website is www.advancedanimalimaging.com
AMES, IA: The Lloyd Veterinary Medical Center at Iowa State University offers a mini-scan MRI package at approximately $800.00, consisting of an examination, MRI scan, and anesthesia. To schedule an appointment, call the neurology service at 515-294-4900. It is located at 1600 S. 16th Street, Ames IA 50011, and its website is vetmed.iastate.edu/vmc
OVERLAND PARK, KS: Dr. Brian C. Cellio, board certified veterinary neurologist at Veterinary Specialty and Emergency Center in Overland Park, Kansas (near Kansas City) offers Cavalier breeders a $900.00 mini-scan MRI per dog for a minimum of five dogs up to ten dogs per day. Contact Dr. Cellio's technician, Mandi, telephone 913-642-9563 or 800-413-6851 to make appointments. The clinic is located at 11950 West 110th Street, Suite B, Overland Park, KS 66210, and its website is www.vseckc.com
COMMERCE, MI: Drs. Michael Wolf, Jared B. Galle, and Andrew Isaacs, board certified veterinary neurologists at Animal Neurology & MRI Center in Commerce, Michigan, offer Cavalier breeders reduced rates as low as $975.00 for an MRI scan. Rate includes a neurological examination, anesthesia, MRI scan and consultation/review of the MRI study with the neurologists, for a minimum of three dogs. Review and MRI imaging report from their board certified radiologist can be requested for an additional fee. Contact Dr. Wolf at the Animal Neurology & MRI Center, 1120 Welch Road., Commerce, MI 48390, Tel: 248-960-7200, email DrWolf@animalneurology.com, website www.animalneurology.com
STARKVILLE, MS: Dr. Andy Shores, board certified veterinary neurologist at Mississippi State University (MSU), announces that the Mississippi State College of Veterinary Medicine is offering a breeding MRI screening program for CKCS and Brussels Griffon breeders. Owners may bring or fax copies of blood and urine analyses from their veterinarian or these tests can be performed on-site (for an additional fee). A complete physical and neurologic examination will be performed as part of the screening process along with a CT metal scan before the MRI. Sedation/anesthesia, including an IV catheter and full and constant monitoring will be provided. A MRI screening (3T magnet: Sagittal T2, Transverse T1 and T2) will be performed. This also screens for primary secretory otitis media (PSOM). The approximate total cost for 2 patients is $1700; however, if MSU screens more than 2 patients, the cost will be approximately $850.00 for each additional patient. The multiple patients screening is designed to accommodate owners with several dogs being considered as part of a breeding program. For the pricing break, MSU needs to screen at least 2 patients at each setting. Contact Dr. Shores' office at 662-325-7339.
COLUMBIA, MO: University of Missouri's College of Veterinary Medicine (Drs. Joan R. Coats, Dennis P. O'Brien, and Fred A. Wininger) offers CM/SM scans for $600.00, which includes "iso" anaesthesia. Blood work is additional and may be obtained ahead of time at your veterinarian's office. Contact Stephanie Gilliam, Neurology/Neurosurgery Technician, University of Missouri Veterinary Medical Teaching Hospital, 900 E. Campus Drive, Columbia, MO 65211, telephone 573-882-7821.
RALEIGH, NC: AnimalScan, at North Carolina State University's College of Veterinary Medicine's Department of Clinical Sciences, 4700 Hillsborough Street, Building 3, Raleigh, NC 27606, telephone 919-838-5209, email raleigh@animalscan.org website animalscan.org
AKRON, OH: Pets Dx Veterinary Imaging, Inc. in Akron, Ohio and Pittsburgh, Pennsylvania, offers a partial MRI, at reduced group rates for Cavaliers, focusing on the head and neck, and includes a compact disc with an imaging program. Their MRIs may be reviewed, for an additional fee, by Dr. Patrick R. Gavin, Diplomate ACVR, Professor of Radiology, Washington State University College of Veterinary Medicine, in consultation with the dog's veterinarian. Pets Dx Veterinary Imaging, Inc. is located at 1321 Centerview Circle, Akron, Ohio 44321, telephone 330-576-6275, and at 807 Camp Horne Road, Pittsburgh, PA 15237, telephone 412-486-4800 and 412-348-2577. Its website is www.petsdx.com
LONDON, ON: Thames Valley Veterinary Services in London, Ontario offers reduced cost MRIs for Cavalier breeders, through the efforts of the CKCS Club of Canada. Current prices (as of June 2014): $475.00 CDN for a mini scan (not including blood work); $575.00 CDN for a full scan (not including blood work).Participants will also receive an MRI that meets all current scanning protocols, a CD copy of their MRI screening, and a report issued by a specialist. Contact Mary Beth Squirrell of the CKCS Canada club for more information, email mesquirrell@gmail.com
PITTSBURGH, PA: Pets Dx Veterinary Imaging, Inc., 807 Camp Horne Road, Pittsburgh, PA 15237, telephone 412-486-4800 and 412-348-2577. See Akron, Ohio entry above for details.
CHARLOTTETOWN, PE: Atlantic Veterinary College at the University of Prince Edward Island offers full MRI scans for $1,40000 CDN (includes HST), blood work not included. Included are a pre-admission neurology exam and full night recovery, radiology and neurology reports. Website avc.upei.ca The CKCS Canada Club contact is susan@wayfinderconsulting.ca
MT PLEASANT, SC: Dr. Peter J. Brofman, (ACVIM Neurology & Internal Medicine) and Veterinary Specialty Care offer a reduced rate for MRIs for breeding screening protocols only. The fee of $1000 includes the MRI and anesthesia but pre-anesthetic blood work is not included. The MRI unit is a 1.5T magnet and is available for screenings Monday through Friday. Veterinary Specialty Care is located at 930 Pine Hollow Rd, Mt. Pleasant, SC 29464. You may contact Dr. Brofman at 843-884-2441, peterbrofman@gmail.com , or www.facebook.com/drpeterbrofman
PURCELLVILLE, VA: Pet MRI & Imaging Services, at Blue Ridge Veterinary Associates, 120 East Cornwell Lane, Purcellville, VA 20132, offers CM/SM mini-scans for $975.00. ( Multiple dogs scanned the same day will be discounted). The clinic has an independent board-certified veterinary Neurologist available to read and report the results to referring DVM within 24 hours. The price includes: IV catheter, anesthesia (typically Propofol induction and inhalant isoflurane), IV contrast, neuro-cranium MRI (multiple views to include sagittal, transverse, dorsal weighted at T1/ T2, etc.), scan review and report by the neurologist, and complete 1-on-1 patient monitoring thru recovery. The owners should be prepared to leave their pets for 3 to 5 hours for preparation, study, and recovery. A report will be emailed to the owner and referring veterinarian. Pre-anesthetic bloodwork (CBC CHEM) should be performed within aprox. 2 weeks of the scan, and may be done by the owner's regular veterinarian. If a patient needs a pre-anesthetic bloodwork panel, the clinic can do so for an additional $98.00. If a patient needs radiographs of the cervical region, those can be done for an additional $95.00. Microchips may be deactivated by the magnet used in the scan. Contact Christy Bell, CPC, LVT, at telephone 540-338-7387 (x104) or 703-606-8516 (work cell), email contact@petmrimaging.com or Blueridgevets@aol.com, website www.PetMRimaging.com
UNITED KINGDOM: See the list of MRI clinics on the website of The Cavalier King Charles Spaniel Club at www.thecavalierclub.co.uk/health/syringo/mriscan.html
NOTE: If you know of other MRI clinics offering reduced rate scans or mini-scans for SM or CM, please let us know by emailing us at Editor@CavalierHealth.org
RETURN TO TOP
DNA Testing
In a March 2018 article, a team of researchers from the UK and Canada examined DNA samples from 65 cavalier King Charles spaniels and narrowed down the likely potential "candidate" genes for CM/SM to PCDH17 in CFA22 (Canis Familiaris Autosome) and ZWINT in CFA26. They used MRI scans of the craniums of affected and normal CKCS to compare size and shape measurements of regions of the brains, to identify lines and angles associated with sizes of syrinx diameters. The PCDH17 gene is involved in the adhesion and sorting of cells in the brain and spinal cord during tissue development. They speculate that the ZWINT gene may be related to neuropathic pain, but this study did not detect any such association. They conclude by calling for additional studies in larger numbers of cavaliers and other affected brachycephalic breeds to investigate the role of the two associated loci and the genes in the pathogenesis of CM/SM.
RETURN TO TOP
Progression
SM is a progressive disease, but its progression can be is extremely variable. Some cavaliers initially may exhibit no scratching or pain; others tend to scratch with only mild pain and no other neurological signs. For some dogs, the initial mild symptoms may never worsen. Other CKCSs can be severely disabled by pain and neurological signs within twelve months of the first signs developing. As the SM syrinx enlarges, it may compress, and in some cases destroy, the surrounding spinal cord tissue.
Chiari-like malformation (CM) also has been found to be progressive in some cavaliers. Interestingly, however, In a January 2025 YouTube video, Dr. Clare Rusbridge observed that as CM-affected cavaliers grow older, their brains shrink, and as a consequence, the severity of fhe CM -- and thus the resulting pain -- may reduce.
Dr. Rusbridge states in her January 2024 YouTube video ("Surgical Options for Syringomyelia") that she has found that for most SM-affected dogs, the growth of the syrinx is rapid at the start and once established, its size and shape remain stable over time. She says that for most dogs, by their third year, they have the biggest syrinx they are going to get.
Dr. Rusbridge stated in 2010, "In our experience, many cases of syringomyelia are not progressive, especially if the syrinx is small." In a 2011 study of 49 cavaliers, the UK researchers found that "the severity of SM was positively correlated with patient age. This is consistent with previous studies indicating that CKCS with SM were significantly older than dogs without SM (Couturier et al., 2008). It seems likely therefore, that SM is a progressive disease in dogs."
Clinical statistics show that about 45% of affected cavaliers develop signs of SM before their first birthday; another 40% will show symptoms between ages one and four years; the 15% balance develop signs later, with the oldest reported case of first developing symptoms at nearly seven years of age.
In a June 2011 study of 555 cavaliers without any symptoms of syringomyelia, 25% of the one year old dogs had SM and 70% of the dogs aged 6 years and older had SM.
In an October 2012 study by UK researchers of 48 cavaliers, nine of which had only CM and the rest had both CM and SM, neuropathic pain progressed in 75% of the dogs over a mean average period of 39 months. The researchers noted that it is not fully understood how CM/SM causes neuropathic pain, and they did not make any such finding.
In a November 2012 UK study by a team of veteran CM/SM researchers of 12 cavalier King Charles spaniels with Chiari-like malformation, they found that all of these conditions increased over time: syrinx width, height of the foramen magnum, length of cerebellar herniation, and caudal cranial fossa volume. The increase in the volume of the cranial fossa is believed to be due to resorption of the supraoccipital bone as syringomyelia progresses. They conclude: "We hypothesise that active resorption of the supraoccipital bone occurs due to pressure from the cerebellum. These findings have important implications for our understanding of the pathogenesis and variable natural clinical progression of CM and syringomyelia in CKCS."
This study confirms a finding in an October 2006 report by Dr. Clare Rusbridge and Penny Knowler that "on post-mortem examination, the supraoccipital bone overlying the cerebellar vermis is remarkably thin and sometimes eroded so that the foramen magnum is enlarged dorsally."
RETURN TO TOP
Treatment
- Drugs
- Physiotherapy -- hydrotherapy
- Electomagnetic field treatment (PEMF)
- Alternative care
- Surgery
- Post-surgery soundwave therapy
The primary goal of treatment is to obtain relief from pain. Treatment options consist of drugs and surgery, as are examined in detail below. Dr. Rusbridge has prepared a flow chart diagram of treatment options (see below), which she calls a treatment algorithm, which is downloadable here in pdf format.
In summary, the first drug to administer, according to this Treatment Algorithm's advice for dogs with either clinical signs of painful Chiari-like malformation (CM-P) or phantom scratching, is gabapentin. For bad pain days, add either an NSAID or paracetamol (Tylenol). Corticosteriods should be reserved as a last resort and never as an initial treatment.
Dr. Rusbridge stated in 2010:
"In our experience, many cases of syringomyelia are not progressive, especially if the syrinx is small, i.e. not every case with this disease needs be managed surgically and many do well on medical management, with drugs that reduce cerebrospinal fluid pressure (e.g. antacids such as cimetidine and omeprazole), non-steroidal anti-inflammatory drugs and adjuvant analgesics such as gabapentin (see Drugs, below). When the surgery for this disease has questionable long-term success it may be more appropriate to treat mild cases medically. As a rule I rarely use corticosteroids when treating spinal cord disease."
RETURN TO TOP
 Drugs
Drugs
- Anticonvulsants & their alternatives, for neuropathic pain
- -- Gabapentin
- -- Pregabalin
- -- Other anticonvulsants
- N-methyl-d-aspartate antagonists (NMDA)
- Anitemetics
- NSAIDs, for non-neuropathic pain from CM
- Proton pump inhibitors
- Antihistamines
- Diuretics
- Carbonic anhydrase inhibitors
- Corticosteroids
- Other drugs
 Treatment
options for CM/SM are very limited. The major means of treating
CM and SM in dogs is called "maintenance" and consists of
medicating the dog with drugs. There is no pharmaceutical
medication that can cure CM or SM. The most that can be hoped
for in using any of these prescription drugs is to attempt to
reduce any types of pain and other symptoms. This is called
"palliative care" and "suppressive care".
Treatment
options for CM/SM are very limited. The major means of treating
CM and SM in dogs is called "maintenance" and consists of
medicating the dog with drugs. There is no pharmaceutical
medication that can cure CM or SM. The most that can be hoped
for in using any of these prescription drugs is to attempt to
reduce any types of pain and other symptoms. This is called
"palliative care" and "suppressive care".
First of all, it is important to distinguish SM with symptoms from SM without symptoms. As a general rule, SM without symptoms (asymptomatic) should not be treated with drugs. Some medications have been effective in some cases were the affected dog shows signs of pain. Neurologists may start treatment with high doses of the drug(s) and then gradually reduce the dosage to the lowest dose which appears able to maintain remission of the symptoms.
(The two photos to the right above are of the same cavalier King Charles spaniel. The photo on the left shows the contorted face of a dog in severe pain. It was taken before oral medication was administered. The photo at the right shows the same dog, after oral medication has taken effect.)
• Anticonvulsants & their alternatives, for neuropathic pain
Anticonvulsants (antiepileptic), such as
gabapentin and
pregabalin, have been successful in
 relieving
neuropathic pain, which is evidenced by behaviors such as
sensitivity to touch or phantom scratching.
relieving
neuropathic pain, which is evidenced by behaviors such as
sensitivity to touch or phantom scratching.
-- Gabapentin
Gabapentin (Neurontin, Gabarone) is an anticonvulsant which works through a receptor on the membranes of brain and peripheral nerve cells. It binds to calcium channels and modulates calcium influx as well as influences GABergic neurotransmission and prevents release of glutamate,the neurotransmitter of pain. Its effect is to deaden the irritated nerve impulses in the dog's neck. In humans, gabapentin reportedly does not interact with any other medications, and it is not metabolized, so it is fully excreted in the urine and has no affect upon the liver. However, in dogs, gabapentin is partially metabolized in the liver, and therefore the prescribing neurologist may be expected to order periodic blood tests to check the liver enzymes.
 Dr. Rusbridge provides a detailed discussion of gabapentin on her
YouTube channel at this link.
Dr. Rusbridge provides a detailed discussion of gabapentin on her
YouTube channel at this link.
In an April 2019 abstract, UK researchers examined the records of 1,415 dogs treated with gabapentin in 2016 for a variety of conditions, including 4.7% for CM/SM. Overall, it was reported to improve clinical signs in 47.3% of the dogs. Most treatments were short-term, with 60% treated for less than a month. The prevalence of suspected adverse reactions was 7.85%, with the most common being sedation (5,2%) and ataxia (1.3%).
In human studies, gabapentin has caused side effects, including sleepiness, dizziness, and leg edema, which were minimized by increasing the dose gradually and by taking the drug with food. In dogs, they may include dose dependant effects, including sedation or drowsiness and recuction in activity. Others may include mild ataxia (loss of coordination) and increased appetite and weight gain. Rare ones include myoclonus and other forms of seizures, vomiting, and diarrhea.
WARNING: Beware of liquid formulations of gabapentin, which may include the sweetener xylitol, which is known to cause profound hypoglycemia and hepatic necrosis in dogs.
The most effective dosage range for Gabapentin is between 10 and 20 mg/kg, starting low, every 8 hours, to maintain effectiveness. Its peak effectiveness is between 1 and 2 hours after the dose. Gabapentin also may be given in combination with NSAIDs. It has been recommended that gabapentin be dosed once every 8 hours, as it lasts in the systems of most cavaliers that long. Gabapentin is commercially manufactured in no less than a 100 mg. capsule. If the dog is prescribed a lower dose than 100 mg and the capsule cannot be split or halved, the drug may have to be specially prepared by a compounding pharmacy.
Gabapentin is not a fast acting medication, taking time to produce results. Also, it may cause ataxia -- loss of coordination of muscular movements.
In an August 2020 article, a team of Colorado State University researchers studied the treatment records of 240 dogs suffering chronic pain due to a variety of diagnosed causes, including osteoarthritis (84.6%), generalized, nonspecific back pain (9.2%), intervertebral disk disease (7.5%), degenerative myelopathy (4.6%), and 56.67% not having a definitive anatomical cause for their pain. None of the dogs had been diagnosed with either Chiari-like malformation or syringomyelia. They report finding that:
"The results from this case series suggest that gabapentin is well-tolerated at much higher doses than what is typically prescribed. Side effects were uncommon, with no clear pattern based on dose, or dog size or age. Therefore, like many other analgesic medications, the efficacy of gabapentin appears patient-specific and should be dosed to effect until side effects are noted or analgesia is achieved."
In a January 2025 YouTube video, Dr. Clare Rusbridge observed that as CM-affected cavaliers grow older, their brains shrink, and as a consequence, the severity of fhe CM -- and thus the resulting pain -- may reduce. Therefore, she advises that the dosages of gabapentin be reviewed periodically in older patients to determine if they need a dose reduction.
RETURN TO TOP
-- Pregabalin
Another anticonvulsant, pregabalin (Lyrica, Accord, Alzain, Lecaent, Milpharm, Prekind, Rewisca, Sandoz, Zentiva), may be prescribed in treating CM/SM once high doses of gabapentin no longer are effective. Pregabalin is about five times more potent than gabapentin and therefore achieves its effect at lower doses (meaning, higher bioavailability). Doses of pregabalin also reportedly have a longer lasting effect than gabapentin. Also, there is no need for a "wash out period" between ending the gabapentin and starting the pregabalin. The starting dose of pregabalin usually is about half the dosage of gabapentin. Some neurologists may skip prescribing gabapentin and start treating with pregabalin (at 4 to 5 mg/kg, twice a day). Pregabalin should have less effect upon the liver than gabapentin, as it is excreted mainly by the kidneys.
In a May 2016 study of six brands of pregabalin, the researchers found that "all brands are pharmaceutically equivalent in their quality aspects." They concluded that the lower cost brands of pregabalin could be used to treat epilepsy. The rearchers do not identify the names of the six brands of pregabalin.
In a July 2019 article, a team of UK researchers divided eight CM/SM-affected, symptomatic cavaliers into two groups -- one administered pregabalin and the other with placebo -- over 10 to 18 days and compared the results using owner questionaire susing a numerical rating scale for pain, quantitative sensory testing, cold latency, and blood samples. They report finding that pregabalin-treated dogs showed:
• improved owner-recorded daily pain scores using a numerical rating scale;
• improved mechanical hyperalgesia (measured sensitivity to pain);
• longer cold tolerance on the neck and humeri.
They concluded that, "Pregabalin was efficacious for the treatment of neuropathic pain caused by Chiari-like malformation and syringomyelia in dogs."
In a November 2019 article, a team of Danish veterinary researchers report on their study of the effects of pregabalin treatment upon 12 cavaliers diagnosed with CM/SM and displaying scratching episodes. The cavaliers were aged from 1 to 7+ years. they were divided into two groups, pregabalin and placebo, and they were treated over a period of 25 days, then no treatment for 2 days, then switched treatment for another 25 days, so that both groups eventually were treated and placebo. They determined the results of the treatment by observing the number of scratching events during 10 minutes of physical activity. They report finding an average of 84% (from 75% to 89%) reduction in the number of scratching events relative to the baseline when compared to the placebo. The dogs' owners assessed the quality of life after treatment as "good" or "could not be better" in 6 of 11 dogs and improved in 4 of 11 dogs. The most prevalent adverse events were increased appetite in 9 of all 12 dogs and transient ataxia (lack of some muscle control) in 9 of the 12 dogs. The researchers concluded that:
"Pregabalin is superior to placebo in the reduction of clinical signs of syringomyelia-related central neuropathic pain in dogs. At a dose range of 13-19 mg kg-1 orally twice daily the encountered adverse events were acceptable to all but one owner."
Dr. Rusbridge provides a detailed discussion of pregabalin on her YouTube channel at this link.
In a January 2025 YouTube video, Dr. Rusbridge observed that as CM-affected cavaliers grow older, their brains shrink, and as a consequence, the severity of fhe CM -- and thus the resulting pain -- may reduce. Therefore, she advises that the dosages of pregabalin be reviewed periodically in older patients to determine if they need a dose reduction
RETURN TO TOP
-- Other anitconvulsants
Paracetamol (acetaminophen, APAP, Tylenol) is another pain reliever, which is a convenient (in most dog owners' medicine cabinets) medication for headaches due to CM. Its mechanism of action is not known. While it is rarely prescribed for dogs, it can be quite useful when appropriately dosed. In large doses, paracetamol can be toxic to dogs' livers.
Topiramate (Topamax) is another anticonvulsant medication which may be prescribed. However, in an August 2015 study, researchers found that no significant quality-of-life difference was observed when cavaliers were treated with either gabapentin or topiramate (Topamax). They also found that their data suggested that the addition of either of these two drugs to dosing of the NSAID carprofen (Rimadyl, Quellin, Vetprofen) may be more effective than carprofen alone. Dr. Rusbridge provides a detailed discussion of topiramate on her YouTube channel at this link, and at this link.
Amitriptyline (Elavil, Tryptizol, Laroxyl, Sarotex) is a tricyclic antidepressant (TCA) by Merck which may be prescribed as an alternative to either gabapentin or pregabalin. In a January 2009 case study of three dogs (none CKCSs), the clinicians report that, "Treatment with the tricyclic antidepressant drug, amitriptyline, or the antiepileptic drug, gabapentin, resulted in either a dramatic improvement or full resolution of clinical signs in all cases." See Dr. Rusbridge's YouTube video on this drug.
Zonisamide (Zonegram) is an anticonvulsant which in clinical trials appears to be effective for generalized seizures in dogs. It's anti-seizure effect is believed to work through sodium and calcium channels. Dr. Curtis Dewey has conducted studies of this drug.
Levetiracetam (Keppra) is an anticonvulsant which can also be used in conjunction with phenobarbital and/or potassium bromide. It appears to be relatively safe for dogs, and reportedly rarely has any adverse side effects and does not appear to affect the liver or liver enzymes.
Oral opioids (pethidine, methadone, tramadol) are alternatives to anticonvulsants. However, there is little evidence of their effectiveness for acute pain in dogs. Methylsulfonyl-methane (MSM) is recommended by some veterinary neurologists as a dietary supplement.
RETURN TO TOP
-- N-methyl-d-aspartate antagonists (NMDA)
Amantadine (Endantadine, Gocovri, Osmolex ER, Symmetrel), is an N-methyl-d-aspartate antagonist (NMDA), which is used for control of the symptoms of Parkinson's disease in humans, together with gabapentin or pregabalin. Amantadine is believed to release brain dopamine from nerve endings making it more available to activate dopaminergic receptors. See this January 2008 article, in which the researchers found clinical improvement in dogs with chronic osteoarthritis pain when amantadine was used in combination with meloxicam. See Dr. Rusbridge's YouTube video on this drug.
Ketamine (Ketalar) is an intravenous or subcutaneously injected NMDA used as an induction and maintenance agent for sedation and to provide general anesthesia. It is being used more frequently in treating CM/SM dogs which no longer respond to gabapentin and pregabalin. It blocks sensory perception in humans. It is administered intraveneously in low doses and infrequently to dogs for CM. It also provides pain relief and controls symptoms of epilepsy, depression, and suicidal thoughts. See Dr. Rusbridge's YouTube video on this drug.
Memantidine (Axura, Ebixa, Namenda) is another NMDA, which is used to treat advanced stages of Alzheimer's disease. See Dr. Rusbridge's YouTube video on this drug.
RETURN TO TOP
-- Antiemetics
Maropitant citrate (Cerenia) is licensed to prevent motion sickness and vomiting (an antiemetic), especially for dogs receiving chemotherapy. Some veterinarians have prescribed it to treat symptomatic syringomyelia, especially phantom scratching. Dr. Rusbridge added maropitant to her CM/SM Treatment Algorithm in 2021 for initial treatment of phantom scratching. Maropitant is an antagonist for the neurokinin (NK1) receptor Substance P, which is present in the dorsal root ganglia and spinal cord dorsal horn.
Dr. Rusbridge says that "Maropitant is amazingly effective at stopping the [phantom] scratching, but the bad news is you can't give it continuously. If you give it continuously, the effect stops. ... I give the owner the box of four tablets for bad scratching days. ... If they are having a particularly bad day, then it may be used then."
In an April 2013 study of 26 cavaliers, German researchers found "an association" between pain and SM asymmetry, and they found "a strong association" between pain and dorsal horn involvement of SM. The researchers conclude that the release of interleukin-6 and substance P is a factor in the development of persistent pain in cavaliers with SM. They suggest that this information could offer new diagnostic and treatment options for CKCSs with SM. An un-published 2021 study of this drug has shown evidence of its safety and effectiveness.
In a January 2021 abstract of a study of this drug treating 9 CM/SM-affected cavaliers, their owners reported that the frequency of phantom scratching had decreased significantly, including spontaneous scratching, scratching while walking, scratching when groomed, and scratching when touched.
See, also Dr. Rusbridge's January 2024 YouTube video on using maropitant for treating phantom scratching.
RETURN TO TOP
-- NSAIDs, for non-neuropathic pain from CM
The use of non-steroidal anti-inflammatory drugs (NSAIDs), such as carprofen* (Rimadyl, Quellin, Vetprofen), meloxicam (Metacam, Loxicom), firocoxib (Previcox), mavacoxib (Trocoxil), grapiprant (Galliprant), and aspirin, may relieve the symptoms of non-neuropathic pain, such as is caused by the Chiari-like malformation (CM) rather than from the syringomyelia (SM), as evidenced by yelping when either being picked up of changing posture. These drugs do not retard deterioration due to progression of the SM.
*WARNING: Carprofen (Rimadyl, Quellin, Vetprofen) may have serious side effects and should not be given without a veterinarian's close guidance and monitoring. Also, carprofen, when combined with omeprazole, may negatively impact intestinal health. See this September 2020 article.
It has been reported that SM-affected cavaliers have been found to have a high level of inflammatory proteins in their bodies, and that for that reason, NSAIDs often provide some initial relief from pain. Also, doxycycline, a tetracycline antibiotic designed to treat bacterial infections, has been prescribed to CM/SM patients as an anti-inflammatory similar to NSAIDs. An alternative to doxycycline is minocycline, which also is an antibiotic in the tetracycline family.
NSAIDs and other conventional analgesic medications have not been found to be effective by themselves to relieve neuropathic pain. Two 2007 studies (1) (2) show that the type of pain behavior suggests that the dogs experience neuropathic pain, probably due to disordered neural processing in the damaged dorsal horn, and that, "as such it is likely that conventional analgesic medication may be ineffective."
NSAIDs should not be administered without supervision by a veterinarian. The US Food & Drug Administration (FDA) warns about the dangers of using NSAIDs here on its website.
RETURN TO TOP
-- Proton pump inhibitors
Drugs which reduce the production of cerebrospinal fluid, including proton pump inhibitors such as omeprazole (Prilosec, Losec, Omesec, Zegerid), and Pantoprazole (Protonix) are reported to be useful to reduce intracranial pressure. (See this April 1997 article: "We conclude that in the canine model, physiological doses of omeprazole decrease CSF production by about 26.") However, in a March 2016 article, researchers reported that, "There was no evidence of an effect of omeprazole on CSF production in healthy dogs." However, in an August 2019 article, the same researchers questioned whether their use of evaluating the albumen quotient (QAlb; ratio between CSF and serum albumin concentration) was an appropriate marker. So, the jury remains out on the effectiveness of omeprazole, but anecdotally, a positive response to omeprazole has been reported. See Dr. Rusbridge's June 2020 article. Dr. Rusbridge also provides a detailed discussion of omeprazole on her YouTube channel at this link.
Long term use of omeprazole is not recommended by some neurologists, as its long term use reportedly has increased the risk of stomach cancer in lab rats. Short term use reportedly can cause a "profound and sustained increase in serum gastrin concentration in dogs." See June 2011 report. As a proton-pump inhibitor, omeprazole inhibits some cytochrome P450 enzymes in humans (primarily CYP2C19) and may inhibit the clearance of some drugs, including diazepam, midazolam, warfarin, and carbamazepine. Omeprazole also reportedly impairs conversion of clopidogrel, an antiplatelet agent, to its active metabolite in humans, leading to decreased antiplatelet efficacy and increased risk for ischemic cardiac events. Omeprazole may also lead to digoxin toxicosis, possibly via inhibition of P-glycoprotein efflux of digoxin. See this June 2013 report. When combined with carprofen, omeprazole may negatively impact intestinal health, according to this September 2020 article.
As a potent inhibitor of gastric acid secretion, all proton pump blockers can decrease the absorption of compounds that require an acidic pH for optimal absorption, including iron supplements, oral zinc, ketoconazole, and itraconazole. This same interaction also applies to H2-blockers (e.g., Pepcid AC [famotidine]). Discontinuing antacids when ketoconazole or itraconazole is being given may be advisable. Alternatively, if antacids cannot be discontinued, fluconazole can be considered if indicated, as fluconazole absorption is not affected by changes in gastric pH. See this June 2013 report.
RETURN TO TOP
-- Antihistamines
Neurologists have been prescribing cimetidine (Tagamet, Zitac), which is a histamine H2-receptor antagonist -- an antihistamine. Histamine contributes to inflammation and causes smooth muscles to constrict. Cimetidine is diffused into the cerebrospinal fluid and reportedly may contribute to reducing the flow of CSF. When taken with gabapentin, cimetidine also reportedly may increase the amount of gabapentin in the blood by decreasing its elimination. Therefore, when taken together, the dosages may require adjustment.
Cimetidine is a potent inhibitor of several families of cytochrome P450 enzymes and can also inhibit transporter pumps and decrease the renal excretion of some drugs, including clearance of many drugs, such as theophylline, lidocaine, midazolam, and propranolol. See this June 2013 report. Dr. Rusbridge provides a detailed discussion of cimetidine on her YouTube channel at this link.
Another H2-blocker occasionally prescribed is famotidine (Pepcid AC). Dr. Rusbridge provides a detailed discussion of famotidine on her YouTube channel at this link.
RETURN TO TOP
-- Diuretics
Diuretics furosemide (Lasix, Diuride, Frudix, Frusemide) and spironolactone (Aldactone), also reduce the production of cerebrospinal fluid and are reported to be useful to reduce intracranial pressure and to slow the progression in the size of the SM ysrinxes. Diuretics are being used infrequently, due to side effects and the success of other CSF pressure reducers. Dr. Rusbridge provides a detailed discussion of furosemide on her YouTube channel at this link.
In an April 2016 article, a UK veterinary neurologist reported studying the effect of furosemide therapy upon the progression of syrinx growth in seven cavalier King Charles spaniels. He stated:
"In all seven dogs, syrinx width and length increased at the follow-up MRI and in four cases a new syrinx had developed (Table 1). Furosemide did not prevent further syrinx expansion nor reduce the size of the syrinx but it remains unknown whether the medical treatment may have delayed the inevitable expansion of the syrinx. Studies of a larger population and prospective, randomised, blinded comparisons between different treatments (medical, surgical, medical vs surgical) are needed to ascertain which will produce the best clinical results."
In a July 2025 study of the progression of SM in Pomeranians, 12 of 45 of them were treated with furosemide during the interval between their first and second MRI scans. The researchers found that the increase in the diameter of the SM at the time of their second MRI "was significantly smaller in dogs treated with furosemide compared to those not treated."
Natural diuretics include urea (AC Carbamide) by Standard Process, and Wu Ling San by Mayway and Alisma by Seven Forests, both traditional Chinese herbal medicines (TCM). Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained in TCM. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
RETURN TO TOP
-- Carbonic anhydrase inhibitors
Carbonic anhydrase inhibitors, such as acetazolamide (Diamox) also serve to decrease the flow of cerebrospinal fluid, but their adverse side effects of abdominal pain, lethargy, weakness, and bone marrow suppression limit long term use. Dr. Rusbridge provides a detailed discussion of acetazolamide on her YouTube channel at this link.
Methazolamide (Glauctabs, MZM, Neptazane), also is a carbonic anhydrase inhibitor. Carbonic anhydrase is a protein which can affect fluid production in various parts of the body. Methazolamide reduces the activity of this protein. It's initial use was to treat glaucoma by reducing the amount of fluid produced in the eyes and therefore also reducing pressure in the eye.
RETURN TO TOP
-- Corticosteroids
Before the disease progresses to its severe form, the use of anti-inflammatory corticosteroids, such as prednisolone (Prelone, Prednidale), methyl-prednisolone (Medrol, Medrone), and dexamethasone (Decadron, Dexamethasone Intensol, Dexone, Hexadrol), may relieve the symptoms but not the deterioration. As a general rule, corticosteroids should be reserved for a last resort, although some neurologists will start initial treatment of symptomatic dogs with a combination of an anticonvulsants, such as gabapentin, and a none-inflammatory corticosteroid. However, dogs should never be treated with both a cortisteroid and an NSAID at the same time. See this March 2011 report.
All that said, however, Dr. Rusbridge stated in July 2024 that she is least likely to use steroids, and, "some dogs come on steroids to my clinic, and the first thing we do is remove the steroids, and the dogs are much better for it." In an April 2025 YouTube video, Dr. Rusbridge said (at 49:31 minutes):
"At the current time, I see hundreds and hundreds and hundreds of dogs with Chiari-malformation and syringomyelia. I only have two dogs that are receiving corticosteroids, and both of those dogs, the corticosteroids are not helping those dogs, and the main challenge is to get both of those dogs off of the corticosteroids. And in both instances, those dogs are having to use ketamine to help reduce the dogs' pain to allow us to reduce the corticosteroids. Please do not blanket treat these dogs with corticosteroids! You are doing more harm than good. And I can't overstate that enough!"
Corticosteroids have serious side effects, such as weight, gait, and skin changes, harmful suppression of the immune system, and iatrogenic hyperadrenocorticism (Cushing's disease). Long term use of these drugs is not advised. Dr. Rusbridge provides a detailed discussion of corticosteroids on her YouTube channel at this link.
RETURN TO TOP
-- Other drugs
Oclacitinib (Apoquel): In a June 2017 article, a German internal medicine specialist treated two cavalier King Charles spaniels with the main symptom of "nonspecific itching", both diagnosed by MRI to have Chiari-like malformation and syringomyelia, with oclacitinib (Apoquel), a selective inhibitor of janus kinase (JAK1 and JAK3) enzymes, which are proteins involved in signaling a pathway that results in itching and inflammation. She stated that, in the case of allergic dermatitis, oclacitinib inhibits itching in the neurological range. She reported:
"In the two Cavalier King Charles Spaniels presented, a therapy with oclacitinib was initiated in the standard dose, which is recommended for atopic dermatitis (0.4-0.6 mg / kg 2 × daily for 14 days, then 1 × daily administration). ... Result and conclusion: After only 2 days the itching is clearly reduced, no scratching behavior can be observed after 1 week and the owners report a generally improved general condition. Both patients are taking the drug for a year now and are clinically inconspicuous, side effects have not occurred."
Melatonin is a hormone produced by the pineal gland in the brain. Melatonin supplement has been prescribed by veterinarians to ease anxiety and restlessness in dogs. Some vets also have prescribed it for dogs with SM symptoms. Since melatonin is a hormone, it should not be given to dogs without the advice of a veterinarian. Melatonin can have adverse side effects, and in particular, it is contraindicated for dogs that are pregnant or lactating.
Maropitant citrate (Cerenia) is licensed to prevent motion sickness and vomiting (an antiemetic), especially for dogs receiving chemotherapy. Some veterinarians have prescribed it to treat symptomatic syringomyelia, especially phantom scratching. Dr. Rusbridge added maropitant to her CM/SM Treatment Algorithm in 2021 for initial treatment of phantom scratching. Maropitant is an antagonist for the neurokinin (NK1) receptor Substance P, which is present in the dorsal root ganglia and spinal cord dorsal horn. In an April 2013 study of 26 cavaliers, German researchers found "an association" between pain and SM asymmetry, and they found "a strong association" between pain and dorsal horn involvement of SM. The researchers conclude that the release of interleukin-6 and substance P is a factor in the development of persistent pain in cavaliers with SM. They suggest that this information could offer new diagnostic and treatment options for CKCSs with SM. An un-published study of this drug has shown evidence of its safety and effectiveness. However, no studies of treating SM-affected dogs with Cerenia have been published.
RETURN TO TOP
 Physiotherapy -- hydrotherapy
Physiotherapy -- hydrotherapy
Physiotherapy, particularly hydrotherapy (right), has been found to be the best form of treatment for scoliosis and other gait abnormalities. The goal is to mazimize their musculature to enable the affected dogs to best compensate for the progression of CM/SM. Surgery is the back up option for treating this category of symtomatic dogs.
RETURN TO TOP
Electomagnetic field treatment (PEMF) -- Assisi Loop
Targeted pulsed electromagnetic field (PEMF)
treatments have been studied and used on dogs
 following decompressive
spinal surgery. A device which creates a pulsed electromagnetic field is
placed near the affected area of the dog's body produces a small,
localized electrical field surrounding the dog's affected, inflammed
tissues. This reportedly increases calcium binding by calmodulin, which
binds to constitutive nitric oxide synthase (cNOS), producing nitric
oxide (NO) with a vasodilatory effect encouraging blood-flow, and
limiting inflammation, and increasing bone growth and repair. Recently,
this device also has been used with some reported anecdotal success in
treating pain in CM/SM dogs.
following decompressive
spinal surgery. A device which creates a pulsed electromagnetic field is
placed near the affected area of the dog's body produces a small,
localized electrical field surrounding the dog's affected, inflammed
tissues. This reportedly increases calcium binding by calmodulin, which
binds to constitutive nitric oxide synthase (cNOS), producing nitric
oxide (NO) with a vasodilatory effect encouraging blood-flow, and
limiting inflammation, and increasing bone growth and repair. Recently,
this device also has been used with some reported anecdotal success in
treating pain in CM/SM dogs.
In an
August 2018 article, North Carolina State veterinary school
researchers reported
 that a loop PEMF device resulted in evidence of
reduced incision-associated pain in post-IVDD-surgeries and may also
have reduced the extent of spinal cord injury and enhanced gait and
movement function. In November 2018, NC State University's board
certified veterinary neurologist Dr. Natasha Olby commenced a study of
cavaliers diagnosed with CM/SM and exhibiting signs of pain and phantom
scratching for the effect of PEMF treatments to control the pain and
itching sensations. The treatments were to be delivered in the form of a
PEMF loop (see image above at left) for a period of two months. The loop is marketed
as the Assisi Loop to veterinarians and pharmacists.
that a loop PEMF device resulted in evidence of
reduced incision-associated pain in post-IVDD-surgeries and may also
have reduced the extent of spinal cord injury and enhanced gait and
movement function. In November 2018, NC State University's board
certified veterinary neurologist Dr. Natasha Olby commenced a study of
cavaliers diagnosed with CM/SM and exhibiting signs of pain and phantom
scratching for the effect of PEMF treatments to control the pain and
itching sensations. The treatments were to be delivered in the form of a
PEMF loop (see image above at left) for a period of two months. The loop is marketed
as the Assisi Loop to veterinarians and pharmacists.
See this YouTube video about the Assisi Loop treating a cavalier, Madison (right), diagnosed with CM/SM. For more information, see the Assisi Animal Health website.
RETURN TO TOP
Alternative care
Various types of what are called "complementary therapies" are helpful to affected dogs, particularly to relieve pain. In addition to hydrotherapy and the Assisi Loop mentioned above and those treatments listed below in this section, choices include gentle massage and transcutaneous electrical nerve stimulation (TENS) -- a small device that delivers the current at or near your nerves to block or change your perception of pain, and ultrasound therapy. However, spinal manipulation, such as by chiropractic, is not appropriate for CM/SM patients, warns Dr. Clare Rusbridge.
Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained. Search webpages for finding holistic veterinarians in the United States are located here and here.
-- Cannabinol (CBD oil)
 There are no published research articles focused upon
cannabinol (CBD) treating
neuropathic pain. Nontheless, veterinary neurologists have considered
the possible value of CBD products in treating forms of neuropathic
pain, including pain due to Chiari-like malformation and/or
syringomyelia. CBD oil might have some use for managing pain through the
cannabinoid CB2 receptors, according to veterinary neurologist Dr. Clare
Rusbrige.
There are no published research articles focused upon
cannabinol (CBD) treating
neuropathic pain. Nontheless, veterinary neurologists have considered
the possible value of CBD products in treating forms of neuropathic
pain, including pain due to Chiari-like malformation and/or
syringomyelia. CBD oil might have some use for managing pain through the
cannabinoid CB2 receptors, according to veterinary neurologist Dr. Clare
Rusbrige.
Dr. Rusbridge states in her December 2023 YouTube video "Cannabinol (CBD oil) and neuropathic pain in animals" that it is difficult for her to recommend CBD oil for her patients because:
• What is an effective and safe dose for neuropathic pain? It appears to require a very high dose to compensate for pain.
• Concerns about safety, especially if combined with other medication or given life long. High doses or long term treatement will induce liver enzymes, requiring liver function to be monitored frequently.
• Can be tricky for vets to source and prescribe a legal preparation.
Cannabinol (CBD) is is a cannabinoid compound produced from hemp and marijuana (Cannabis Sativa) plants. CBD oil mimics the endocannabinoid molecules which the dog's (and our) body produces in several different organs. They play roles in reducing pain, regulating inflammation, and affecting the immune system, by initially binding to receptors in the brain.
CBD is non-psychoactive, unlike tetrahydrocannabinol (THC), another cannabinoid compound from marijuana, which is considered psychoactive by altering the mental state, and can be highly toxic to dogs.
Varieties of CBD: Cannabidiol-based veterinary products are derived mainly from hemp (Cannabis sativa) and must contain less than 0.3% tetrahydrocannabinol (THC). This form of CBD can be processed into "full spectrum" or "broad spectrum" and also may be in the form of a "distillate", in which all THC has been removed, or in the form of CBD "isolate", which is a purifed powder.
• Full Spectrum: Full spectrum CBD contains other extracts found in the cannabis plant, including terpenes, and up to 0.3% THC.
• Broad Spectrum: Broad spectrum CBD also contains some other cannabis compounds but no more than trace amounts of THC.
• CBD Isolate: CBD isolate is pure CBD and contains no other cannabis plant compounds.
• Naked CBD: Naked CBD describes CBD oil by itself, as opposed to being capsultated or microcapsulated or combined with any other substance, such as deoxycholic acid (DCA).
• Liposomal CBD: This is an orally administered encapsultated CBD which is packaged within liposomes, small fatty cellular sacs which improve bioavailability of the CBD by enabling it to be withstand digesstion in the stomach and degradation in the liver. Lipsomal CBD was tested on dogs in this September 2020 article.
• Cannabidiolic acid (CBDA) is an a cid precursor of CBD. It forms CBD when heated. It has been shown in some studies to be more potent that CBD for treating rats. It has been found to be more readily absorbed into the human bloodstream than CBD. Aa theory is that adding CBDA to doses of CBD may make the CBD more absorbable. In this September 2020 article, the investigators found that CBDA is absorbed at least twice as well as CBD in dogs within a 24 hour period, with some differences depending upon the medium used to deliver the oral treatment.
The only three classifications of canine disorders for which there have been any published clinical studies are: osteoarthritis, idiopathic epilepsy, and atopic dermatitis. Even those studies have included very few dogs, including as few as 4 dogs for each category of CBD or placebo, and for very short study times. None of these studies rank above "pilot studies".
There are no published research articles focused upon CBD treating neuropathic pain. Nontheless, veterinary neurologists have considered the possible value of CBD products in treating forms of neuropathic pain, including pain due to Chiari-like malformation and/or syringomyelia. CBD oil might have some use for managing pain through the cannabinoid CB2 receptors, according to veterinary neurologist Dr. Clare Rusbrige.
See our Cannabis webpage for additional details about CBD, including delivery methods, bioavailability, dosages, and adverse reactions.
RETURN TO TOP
 --
Nerve Eight (8)
--
Nerve Eight (8)
An herbal supplement which cavalier owners report calms dogs suffering from the symptomatic scratching of SM is a product called "Nerve Eight" or "Nerve 8", manufactured by Nature's Sunshine of Provo, Utah (left), which consists of white willow bark (salix alba), black cohosh root (cimicifuga racemosa), capsicum fruit (capsicum annuum), valerian root (Valeriana officinalis), ginger root (zingiber officinale), hops flowers (humulus lupulus), wood betony herb (betonica officinalis), and devil's claw root (harpago-phytum procumbens).
RETURN TO TOP
-- Palmitoylethanolamide (PEA)
Palmitoylethanolamide (PEA) is a
N-acylethanolamine molecule in a family of long-chain fatty acid
 amides
called ALIAmides. PEA has been found in rat and mice studies to limit
hyperactvity in immune cells and thereby control inflammatory responses
and resulting tissue damage. PEA is produced by the animal's body as
needed in response to certain types of injuries. PEA is a product of
normal fatty acid synthesis from palmitic acid. It is found in many
common foods, particularly palm oil, soy beans, egg yolks, and peanuts.
The commercial version is most commonly manufactured from palm oil*. While no
objective, unbiased clinical studies of the
effect of PEA on treating CM or SM have been published, one
under-powered pilot study, the
April 2011 Normast Study, does exist, and some
veterinarians reportedly are treating these disorders for pain with PEA,
with mixed results.
amides
called ALIAmides. PEA has been found in rat and mice studies to limit
hyperactvity in immune cells and thereby control inflammatory responses
and resulting tissue damage. PEA is produced by the animal's body as
needed in response to certain types of injuries. PEA is a product of
normal fatty acid synthesis from palmitic acid. It is found in many
common foods, particularly palm oil, soy beans, egg yolks, and peanuts.
The commercial version is most commonly manufactured from palm oil*. While no
objective, unbiased clinical studies of the
effect of PEA on treating CM or SM have been published, one
under-powered pilot study, the
April 2011 Normast Study, does exist, and some
veterinarians reportedly are treating these disorders for pain with PEA,
with mixed results.
Not all PEA is alike. There are three types of PEA.
• Basic PEA, called "naive PEA", is almost totally insoluble in water and under gastrointestinal conditions and therefore the oral intake of it (rather than being injected directly into the abdomen) has very poor bioavailability, meaning that it does not get absorbed well in the dog's gut. See this May 2021 article and this July 2025 article.
• Micronized PEA (m-PEA or micro-palmitoylethanolamide) is a patented technique that reduces the diameter of PEA particles, making them absorbable in the intestine, which has been found to be more effective than ordinary basic PEA in activating PEA levels in blood plasma in dogs. See this August 2014 article.
• Ultra-micronized PEA (um-PEA), also patented, reduces the PEA particle size further, to enable it to cross the blood-brain barrier, likewise has been found to be much more effective than basic PEA. See this August 2014 article.
• Water-Dispersible PEA (PEA-WD), also patented, reduces the PEA to a powder which can be dispersed in cold water. It has been found to be 16 times more effective than basic PEA. See this July 2025 article.
• Hybrid versions of PEA: Additonally, PEA has been combined with other ingredients and used in some published studies. These include FenuMat-PEA (P-fen), which is a PEA hybrid combined with the herb fenugreek (trigonella foenum-graecum), and hybrids combined with resveratrol, quercetin, fisstin, daidzein, genistein, and boswellic acid. See this September 2024 article and this May 2025 article and this July 2025 article.
In an August 2014 study comparing the 3 versions of PEA -- basic, micronized, and ultra-micronized -- in treating rats for inflammatory pain, the researchers reported that the pain "parameters were significantly decreased by oral treatment with micronized PEA-m and ultramicronized PEA-um at each time point compared to nonmicronized PeaPure."
In a July 2025 article. the researchers compared the dissolution rate and bioavailability of basic PEA and water-dispersible PEA (PEA-WD )and reported finding that PEA-WD was 16 times more effective than basic PEA.
PEA micronization and ultra-micronization are patented (by Italian
company, EPITECH
Group SpA) processing techniques that reduce the diameter of the PEA
particle to a micronized or ultra-micronized size
 which optimizes the
PEA's absorbability along the intestine. Micronization increases the
drug's surface area, thereby improving its dissolution rate and
minimizing its absorption difficulties. The ultra-micronized size
also enables
the PEA to cross the blood-brain barrier. See this
February 2021
article.
which optimizes the
PEA's absorbability along the intestine. Micronization increases the
drug's surface area, thereby improving its dissolution rate and
minimizing its absorption difficulties. The ultra-micronized size
also enables
the PEA to cross the blood-brain barrier. See this
February 2021
article.
If a PEA product is not advertised as being micronized or ultra-micronized (or water-dispersible), then Dr. Clare Rusbridge advises that "You probably are wasting your money." A variety of brands of micronized and ultra-micronized PEA are offered on-line.
Recent research has produced evidence that ALIAmides can relieve dogs with hypersensitive skin disorders. In an August 2015 article, Italian researchers conducted an 8-week study of the effectiveness of oral ultra-micronized palmitoylethanolamide (um-PEA) in 160 dogs with moderate atopic dermatitis. Each dog received a daily dose of um-PEA at the rate of 10 mg/kg for 56 days. They report finding that um-PEA appeared to be effective and safe in reducing pruritus and skin lesions, and in improving the quality of life in dogs with moderate atopic dermatitis and moderate pruritus.
As for dosages, the studies using micronized PEA, the range was from 10 to 15 mg/kg/day, and the range for ultra-micronized was 24 mg/kg (for osteoarthritis).
Read more about PEA on our PEA webpage.
* Palm oil: The palm oil cultivation industry has been destroying rainforests in Sumatra and Borneo in Indonesia and Malaysia, the only habitats of orangutans. If you are going to obtain PEA, we suggest that you do so only from vendors whose PEA has been manufactured with palm oil from sustainable sources and not the deforestation of rainforests. This link connects to a "PalmOil Scan Mobile App" which will enable you to determine if the PEA vendors you select obtain their palm oil from sustainable sources.
RETURN TO TOP
• Acupuncture
In a
September 2016 article, a UK veterinarian reported on the successful use
of acupuncture (photo at right) to control the
signs of pain in a cavalier King Charles spaniel suffering from Chiari-like
malformation and
 syringomyelia (CM/SM). The acupuncture was in addition to
conventional medical treatment. He noted:
syringomyelia (CM/SM). The acupuncture was in addition to
conventional medical treatment. He noted:
"This patient exhibited signs which suggested it was also suffering from headaches. It often presented with a frowning expression and during these times intensely disliked being touched, rubbed or patted on the head. Acupuncture had a definite positive effect on this patient with reductions in all the signs including the signs of phantom scratching and vocalisation."
Dr. Curtis Dewey, neurologist at Cornell University, is conducting a study (2019-2020) to evaluate the effectiveness of acupuncture treatments on the thermal patterns in the cervical region of cavalier King Charles spaniels with Chiari-like malformation (CM) and syringomyelia. Ten CKCSs will be in each of three groups -- one treated with dry needling acupuncture, one with electro-acupunture, and the third a placebo group. Thermal images taken before and after treatments will be compared. The dogs' owners will also be asked to complete questionnaires. Details are on this webpage.
• Laser therapy
Cold laser therapy, including K-Laser (high power laser therapy) and LZ30-z (frequency-specific low level laser therapy) has been used anecdotally with some reports of successfully relieving signs of pain and discomfort when applied periodically.
RETURN TO TOP
Surgery
- Introduction
- Foramen Magnum Decompression (FMD)
- Cranioplasty using titanium
- Cranioplasty using LactoSorb SE mesh
- Duraplasty using swine or bovine tissue
- Duraplasty using only patient's fat tissue
- Duraplasty using tissue adhesives
- Syringosubarachnoid shunt
- Ventriculoperitoneal shunt
- Conclusion about surgeries
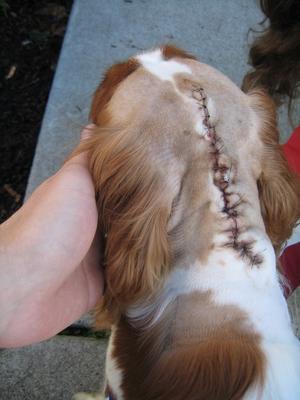 •
Introduction to Surgery
•
Introduction to Surgery
Surgery to allow the cerebrospinal fluid to flow normally may be necessary to reduce the pain and deterioration. Surgery is directed only at the Chiari-like malformation. It is not upon the syrinx, although it should eliminate growth of the syrinx. Surgery is recommended if there is significant pain or a deteriorating condition despite medications and alternative therapies.
The current threshold for surgery seems to consist of dogs with: (1) MRI evidence of Chiari-like malformation and cervical syringomyelia; (2) Syrinx in the cervical spinal cord measuring >3mm diameter on transverse T2 MRI; nd (3) Clinical signs of phantom scratching, cervical pain or hypersensitivity, or thoracic limb paresis without MRI/CSF evidence of other changes that could produce the same clinical signs.
Surgery usually is successful in significantly reducing the Chiari-pain and improving the neurological deficits. However, surgery will not always reverse the filling mechanism of the syrinx and cause it to collapse. The extent of a successful outcome of a surgery is best determined by a post-operative MRI scan at around six months.
Not all neurologists experienced with CM and SM in cavaliers agree about when and if surgery should be performed. Some have found that early surgical treatment is more successful than waiting and considering it as a last resort, and that the longer the dog has been in pain, the less likely it will recover. However, others have observed that SM will not continuously progress to a severe stage in all SM-affected young dogs.
Dr. Andy Shores of Mississippi State University has stated that, "Severely affected patients with syrinxes <3mm in diameter that respond poorly to medical management might also benefit from surgery."
The earliest reported surgeries to relieve CM was a series of suboccipital craniectomies and cranial dorsal laminectomies by a team of Belgium neurosurgeons in 2003. They had little success. Read their report here.
Terms which describe different types of surgical procedures include craniectomy, cranioplasty, duraplasty, and insertion of a shunt:
• Craniectomy involves removing a portion of the skull bone to relieve pressure. This procedure is common in most surgeries treating CM/SM.
• Cranioplasty begins with a craniectomy, followed by replacement of the removed portion of bone with a titanium or a synthetic material. It includes foramen magnum decompression (FMD) described below.
• Duraplasty is the name of a surgical procedure which grafts a piece of fat or synthetic tissue to the portion of the dog's dura (the fibrous membrane beneath the skull, which insulates the brain and central nervous system), which must be cut during these surgeries.
• Shunt is the insertion of a tube, usually silicone, into the syrinx, to drain fluid from the area around the syrinx and thereby reduce the size of the syrinx.
Dr. Rusbridge provides a detailed discussion of CM/SM surgery on her YouTube channel at this link.
RETURN TO TOP
• Foramen magnum decompression (FMD)
The most common form of surgery is called foramen magnum
decompression (FMD), or suboccipital decompression
or cranial cervical decompression, surgery. The surgeon removes the
supraoccipital bone and the cranial dorsal laminae of the
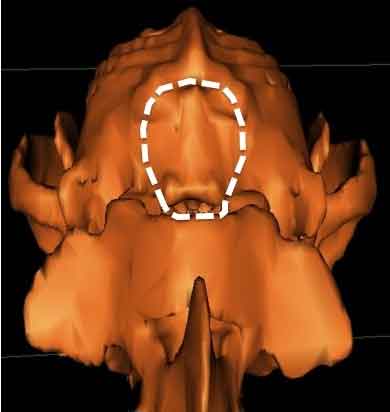 atlas.
(See the decompression site over the occipital bone and foramen magnum,
outlined in diagram at right.) Decompression surgery may include incising through the dura sac, a tough
membrane which contains the brain inside of the skull, and installing a
dural graft or shunt, to allow more space for the cerebellum and to reduce
the pressure of the flow of CSF. In some surgeries, the entire
occipital bone also is removed. All FMD surgeries are technically
difficult and should be performed only by experienced neurological surgeons.
atlas.
(See the decompression site over the occipital bone and foramen magnum,
outlined in diagram at right.) Decompression surgery may include incising through the dura sac, a tough
membrane which contains the brain inside of the skull, and installing a
dural graft or shunt, to allow more space for the cerebellum and to reduce
the pressure of the flow of CSF. In some surgeries, the entire
occipital bone also is removed. All FMD surgeries are technically
difficult and should be performed only by experienced neurological surgeons.
Although this form of surgery often is successful in relieving pain due to Chiari, it may be expensive, and many dogs may either have a recurrence of the disease or still show some signs of pain and phantom scratching. Some post-operative pain is only temporary, due to leakage of CSF through the incision in the dura until that incision heals, or because the syrinx is still present after the surgery. However, medications which did not work prior to surgery may be more successful following it. Nevertheless, since phantom scratching is due to the SM syrinx and not the Chiari-like malformation, it may be expected to continue following this form of surgery.
The most frequent reason for recurrence reportedly is the development of post-operative scar tissue which compresses the cervicomedullary junction. Scar tissue has required additional surgery to remove it in about 25% of all FMD surgical cases. To avoid the development of scar tissue, it is important to not allow the dog too much freedom of movement or excitement during the healing process, which may last from three to six months.
In an effort to prevent such scar tissue from re-compressing the junction, modified versions of FMD include inserting either a skull plate made of titanium mesh at the junction before closing the incisions, (see photo below), or covering the sutured dura with a tented graft of swine intestine tissue, covered by a layer of the dog's fat tissue.
In a November 2025 article, Korean veterinary surgeons Sung Su Park,
Ji Young Park, and Ho Jae Han report the results on performing foramen
magnum decompression surgery on 87 small dogs (none being cavaliers)
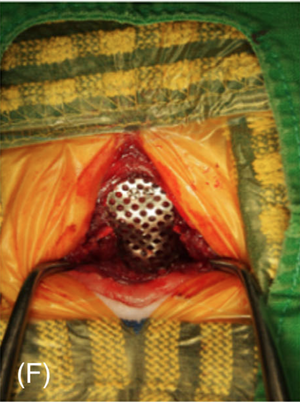 diagnosed
with Chiari-like malformation and syringomyelia (CM/SM), using a
titanium mesh (TM) shaped with a gap between the surface of the
cerebellum. Their aim was to avoid the formation of post-operative scar
tissue due to the TM coming into contact with the cerebellum in dogs
with limited size of the occipital region. The TM plate they designed is
contoured to follow the occipital curvature, with a decompression gap
over the cerebellum and also avoiding contact with the dorsal arch of
the first cervical vertebra. (See photograph.) A total of 76
dogs (87%) showed long-term improvement, with no follow-up surgeries
required. A subset of 11 dogs (13%) required continued medication after
surgery due to the recurrence of signs. They concluded that:
"Retrospective analysis showed that the modified TM technique, using a
deliberate gap, was safe and effective in small-breed dogs with CM/SM,
successfully preserving the decompression space and maintaining
long-term neurologic stability."
diagnosed
with Chiari-like malformation and syringomyelia (CM/SM), using a
titanium mesh (TM) shaped with a gap between the surface of the
cerebellum. Their aim was to avoid the formation of post-operative scar
tissue due to the TM coming into contact with the cerebellum in dogs
with limited size of the occipital region. The TM plate they designed is
contoured to follow the occipital curvature, with a decompression gap
over the cerebellum and also avoiding contact with the dorsal arch of
the first cervical vertebra. (See photograph.) A total of 76
dogs (87%) showed long-term improvement, with no follow-up surgeries
required. A subset of 11 dogs (13%) required continued medication after
surgery due to the recurrence of signs. They concluded that:
"Retrospective analysis showed that the modified TM technique, using a
deliberate gap, was safe and effective in small-breed dogs with CM/SM,
successfully preserving the decompression space and maintaining
long-term neurologic stability."
Decompression surgery is not expected to cure the SM. It is intended to reduce the pressure and stop the progression of the syrinxes. Damage done to the brain and spinal cord before the surgery usually will not be reversed, and most dogs will need to continue on medications afterwards, including gabapentin or pregabalin and cortisteroids, depending upon the severity of that damage before the surgeries. The neurologists also may recommend that the post-surgery patient undergo rehabilitation physical therapy, in part to offset debilitating effects to the muscles, which may result from long term doses of cortisteroids.
RETURN TO TOP
• Cranioplasty using titanium
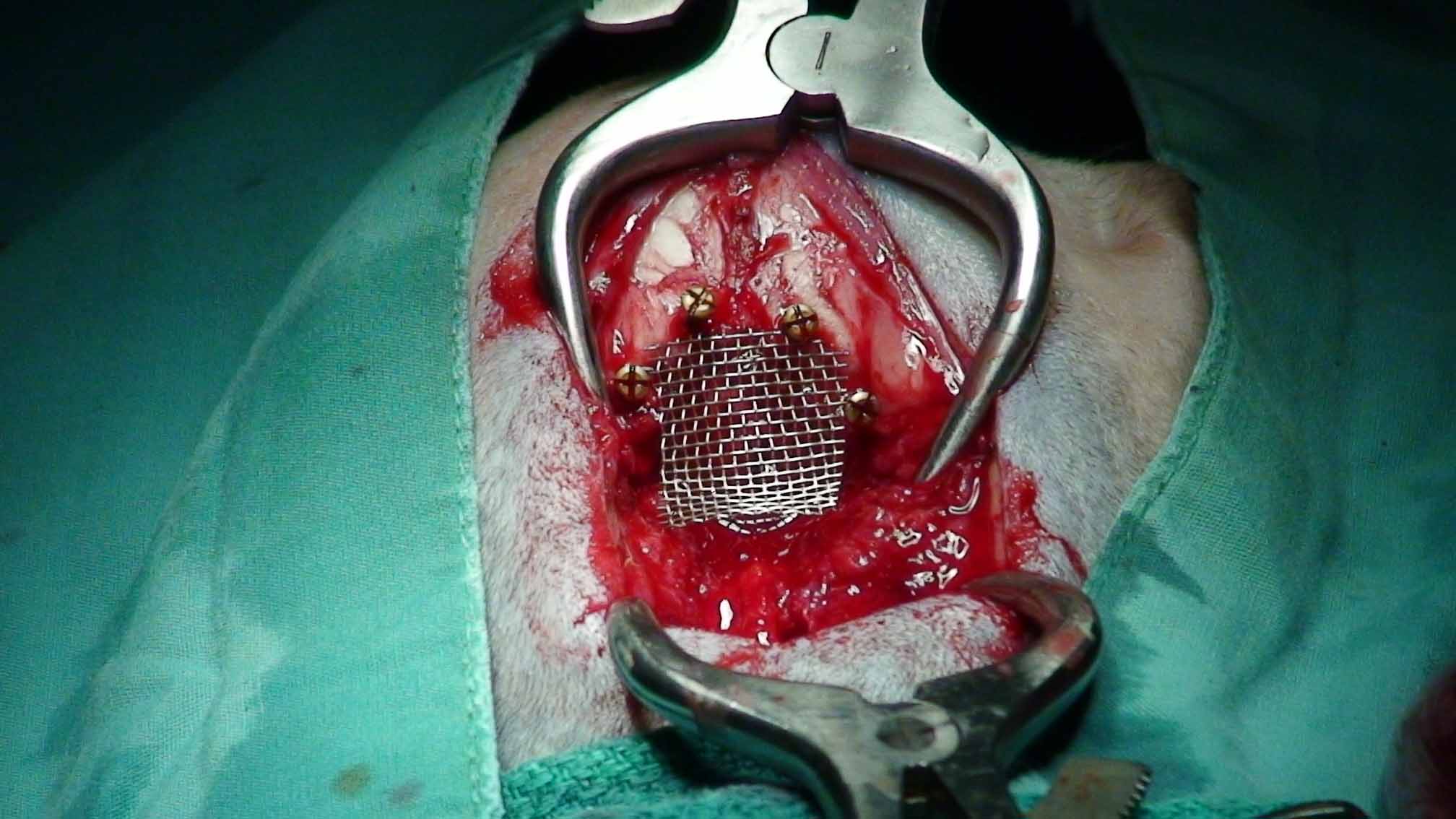 Cranioplasty begins with a foramen magnum decompression (FMD) described
above, followed by replacement of the removed portion of bone with a
titanium or a synthetic material.
Cranioplasty begins with a foramen magnum decompression (FMD) described
above, followed by replacement of the removed portion of bone with a
titanium or a synthetic material.
The titanium procedure involves inserting titanium mesh to cover the wider opening created by the FMD precedure. (See image at right.)
In a report published in July 2007 in Veterinary Surgery, Drs. Curtis W. Dewey and Dominic J. Marino wrote:
"Foramen Magnum Decompression (FMD) with cranioplasty was well tolerated, with no intraoperative complications, and minor postoperative complications. Most dogs improved clinically, and none required further surgery at the original FMD site."
Dr. Dewey also has reported that the "re-operation rate" has been reduced to 10% or less of all FMD surgeries with the titanium mesh cranioplasty. See more about Drs. Dewey and Marino under Current Research below. Dr. Thomas Schubert, formerly at the University of Florida, has applied a calcium-based bone cement over the porous titanium mesh to further prevent scarring.
In
a
February 2022 article, Italian neuro-surgeons reported on the use
of a customized 3-D-printed titanium prosthesis (device) as occipital
cranioplasty to cover the bone defect and enlarge the caudal fossa
(instead of the more traditional titanium mesh), following foramen
magnum decompression (FMD) in 8 CM/SM-affected dogs, including 6
cavaliers (75%). Each prosthesis was customized by
using computed tomography (CT) of the dog's skull, followed by 3-D
reconstruction of the skull to design the prosthesis. (See photos of
one of the 3-D reconstructed skulls below.) FMD was performed, and
the prosthesis was implanted in each case. Follow-up examinations were
performed 1, 6, and 12 months later, and the clinical status of each
dog's recovery was graded. Repeated MRI images of 5 of the cases were
compared to identify changes involving the neural
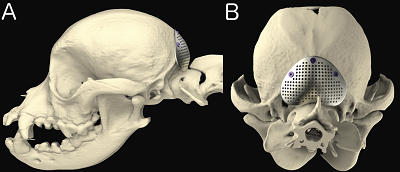 structures,
particularly the syrinx. The investigators reported that all prostheses
were easily positioned based on the pre-operative 3-D models, with no
complications. At 12 months after surgery, 3 cavaliers were free of
previous medications, and 2 CKCSs were still receiving steroid
medications but at lower doses. MRI of 4 cavaliers 6 to 20 months after
surgery revealed resolution of SM in CKCS Case 7, reduced size of SM in
CKCS Cases 2 and 4, and worse SM in CKCS Case 6. Details of each of the
6 cavalier cases
is reported here.
structures,
particularly the syrinx. The investigators reported that all prostheses
were easily positioned based on the pre-operative 3-D models, with no
complications. At 12 months after surgery, 3 cavaliers were free of
previous medications, and 2 CKCSs were still receiving steroid
medications but at lower doses. MRI of 4 cavaliers 6 to 20 months after
surgery revealed resolution of SM in CKCS Case 7, reduced size of SM in
CKCS Cases 2 and 4, and worse SM in CKCS Case 6. Details of each of the
6 cavalier cases
is reported here.
RETURN TO TOP
• Cranioplasty using LactoSorb SE mesh
LactoSorb SE mesh is a biodegradable polymer designed to resorb in the human body by hydrolysis within a year. Dr. Thomas Schubert at the University of Florida has tried it instead of titanium mesh in cranioplasty surgeries performed on cavaliers. He since has switched back to using the titanium mesh. The polymer reportedly is equal in strength to titanium at initial placement, retains 70% of its initial strength for the first eight weeks, and then gradually is eliminated from the body. It is manufactured by Biomet Microfixation, LLC of Jacksonville, Florida.
RETURN TO TOP
• Duraplasty using swine or bovine tissue
The alternative of a tent graft of swine or bovine collagen tissue and body
fat is called duraplasty.*
Dr. Andy Shores,
veterinary
 neurologist at
Mississippi State University (previously at Auburn in Alabama), Dr. Jill
Narak, veterinary surgeon at the University of Tennessee in
Knoxville, and others have performed this procedure on dozens of dogs,
most all of them cavaliers. Swine
intestinal submucosa was sutured over the cerebellum and brain stem in a
tented fashion (see photo). Fat tissue from the dog's gluteal region was then placed
over the site prior to routine closure.
neurologist at
Mississippi State University (previously at Auburn in Alabama), Dr. Jill
Narak, veterinary surgeon at the University of Tennessee in
Knoxville, and others have performed this procedure on dozens of dogs,
most all of them cavaliers. Swine
intestinal submucosa was sutured over the cerebellum and brain stem in a
tented fashion (see photo). Fat tissue from the dog's gluteal region was then placed
over the site prior to routine closure.
* It is called duraplasty because the tissue is used as a substitute to the portion of the dura which was cut during the surgery.
In a report published in October 2009 at the American College of Veterinary Surgeons' annual symposium, the researchers stated: "Overall, recovery was considered to be good to excellent by owners. To date, none of the patients that have undergone this surgical procedure have required further surgical intervention due to postoperative compressive scar formation that has been reported in the previous literature. Follow-up time ranges from 1 week to 1 year. ... The use of the titanium mesh, placement of the screws, and the exothermic reaction of the overlying methyl methacrylate may contribute to tissue trauma. The authors conclude that with the results of this study, this procedure is clinically effective and the use of a titanium mesh, additional hardware and methyl methacrylate offers no advantage in canine COMS patients."
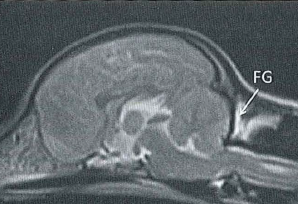 In a
March 2015 report, Dr. Andy Shores and his surgical team at
Mississippi State University examined the results of 23 such duraplasty
surgeries they had performed, including 18 CKCSs. They noted that all of the dogs'
conditions had improved. Of 17 of the dogs, whose owners returned
surveys at least a year later, no dog has required additional surgery,
all but one had some improvement in quality of life after surgery, and
none were judged to deteriorate to less than the pre-surgical status. (In
the MRI scan at right, taken a year after swine duraplasty surgery,
shows the location of the swine fat graft [FG]. Courtesy of Dr. Shores.)
In a
March 2015 report, Dr. Andy Shores and his surgical team at
Mississippi State University examined the results of 23 such duraplasty
surgeries they had performed, including 18 CKCSs. They noted that all of the dogs'
conditions had improved. Of 17 of the dogs, whose owners returned
surveys at least a year later, no dog has required additional surgery,
all but one had some improvement in quality of life after surgery, and
none were judged to deteriorate to less than the pre-surgical status. (In
the MRI scan at right, taken a year after swine duraplasty surgery,
shows the location of the swine fat graft [FG]. Courtesy of Dr. Shores.)
In a December 2017 article, a team of Korean veterinary surgeons report the successful grafting of Lyoplant, a pure collagen implant that is produced from bovine pericardium, as a substitute for the portion of the brain's dura removed during foramen magnum decompression surgery of a Maltese dog diagnosed with Chiari-like malformation, and syringomyelia. They conclude that "surgical decompression with Lyoplant was an effective long-term (12-month) treatment for COMS without the need for any pharmacological treatment."
RETURN TO TOP
• Duraplasty using only patient's fat tissue
In a 2015 case study, a team of Korean surgeons report the successful duraplasty procedure on four CM/SM-affected dogs (breeds unidentified) using only the patients' own fat tissue.
RETURN TO TOP
• Duraplasty using tissue adhesives
Synthetic dural substitutes in duraplasty procedures include inserting a soft foam consisting of a collagen-based matrix (e.g., DuraGen, by Integra LifeSciences Corporation). The collagen matrix supports the ingrowth of local cells while the matrix itself is fully resorbed over time. Dr. Michael Harrington, Animal Neurosurgery and Neurology, Murray, Utah, reportedly uses this technique.
RETURN TO TOP
• Syringosubarachnoid shunt
 Another form of surgery, performed by veterinary neurosurgeon
Geoffrey Skerritt (at right), in the
United Kingdom, and others, involves inserting a shunt, rather than removing the supraoccipital bone or a portion of the atlas. He is said to prefer
the shunt because it reduces the higher risk of nerve damage and blood loss
in decompression surgery, and it lessens the possibility of the cerebellum
continuing to herniate.
Another form of surgery, performed by veterinary neurosurgeon
Geoffrey Skerritt (at right), in the
United Kingdom, and others, involves inserting a shunt, rather than removing the supraoccipital bone or a portion of the atlas. He is said to prefer
the shunt because it reduces the higher risk of nerve damage and blood loss
in decompression surgery, and it lessens the possibility of the cerebellum
continuing to herniate.
The shunt consists of a small silicone tube. One end is inserted into the subarchnoid space of the spinal cord below the syrinx, and the other end is inserted into the syrinx (see below, left). Thus it is called a syringosubarachnoid shunt (S-S shunt). Shunting drains the syrinx fluid into the subarachnoid space where the usual CSF circulation and absorption mechanisms exist. This should reduce the size of the syrinx and ease pain and other the clinical signs associated with CM/SM.
 In
an April 2012 study of S-S
surgeries conducted on nine cavaliers and two Yorkshire terriers, Geoff
Skerritt and Dr. Luca Motta reported that:
In
an April 2012 study of S-S
surgeries conducted on nine cavaliers and two Yorkshire terriers, Geoff
Skerritt and Dr. Luca Motta reported that:
"S-S shunting is a safe and relatively effective surgical technique that may improve the neurological signs and the quality of life of dogs affected by CM and associated SHM/SM. Postoperative complications or lack of clinical improvement may occur in a small number of cases and a secondary surgery may be needed. This study also suggests that the S-S shunt may lead to a satisfactory outcome in dogs where the FMD [foramen magnum decompression] technique has failed. Comparisons between different surgical techniques are needed to create objective criteria that may suggest which procedure will produce the best surgical results."
Mr. Skerritt and Dr. Motta may be contacted at ChesterGates Animal Referral Hospital, Telford Court, ChesterGates, Chester, UK, CH1 6LT, telephone 01244 853823, email GCSkerritt@aol.com.
In a 2018 article, a team of Romanian veterinary neurosurgeons (Cătălina Anca Cucoş, Ateş Barut, Iuliana Ionaşcu, Radu Constantinescu, Constantin Vlăgioiu) report surgically inserting a shunt in a syrinx of a 4-year-old cavalier King Charles spaniel suffering from severe pain and other signs due to Chiari-like malformation and syringomyelia (CM/SM). The dog had undergone cranio-cervical decompression surgery a year before before, which resolved the pain symptoms for three months, followed by progressive deterioration afterwards. For the two months prior to the shunt surgery, the dog's symptoms included neuropathic pain, expressed by sudden yelping, neck and spinal discomfort, scoliosis, apathy, depression, .phantom scratching and bunny-hopping pelvic limb gait.
 The
shunt was fashioned from a ventriculoperitoneal catheter (right)
which was inserted into the syrinx to allow for drainage of
cerebrospinal fluid (CSF). The syrinx was located at C3-C4 and had a
maximum internal diameter of over 2 mm. The clinicians report that the
procedure was successful and that after three years, there remains
notably decreased neuropathic pain, with the intensity and frequency of
pain attacks reduced, scratching and yelping diminished, and overall, an
increased quality of life. They conclude:
The
shunt was fashioned from a ventriculoperitoneal catheter (right)
which was inserted into the syrinx to allow for drainage of
cerebrospinal fluid (CSF). The syrinx was located at C3-C4 and had a
maximum internal diameter of over 2 mm. The clinicians report that the
procedure was successful and that after three years, there remains
notably decreased neuropathic pain, with the intensity and frequency of
pain attacks reduced, scratching and yelping diminished, and overall, an
increased quality of life. They conclude:
"Syringosubarachnoid shunt placement represents a safe and efficient procedure, which leads to clinical improvement of the clinical signs and reestablishes the quality of life. Syringosubarachnoid shunting can be an alternative option with good results, especially in cases in which foramen magnum decompression has failed."
RETURN TO TOP
• Ventriculoperitoneal shunt
A "ventriculoperitoneal shunt" (VPS) is a flexible
tube, called a catheter, one end of which is placed into one of the
lateral ventricles of the dog's brain, and the other end placed much
farther down the dog's body, to
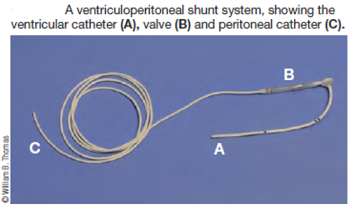 drain
excess CSF away from the brain, where it can be absorbed by the body.
The purpose of shunting is to reduce pressure on the brain caused by the
excess CSF. If the dog has big ventricles (ventricular
dilatation) Dr. Rusbridge states that placing an
intraventricular shunt may be the best option.
drain
excess CSF away from the brain, where it can be absorbed by the body.
The purpose of shunting is to reduce pressure on the brain caused by the
excess CSF. If the dog has big ventricles (ventricular
dilatation) Dr. Rusbridge states that placing an
intraventricular shunt may be the best option.
A shunt (right) consists of three basic parts: (1) a ventricular inflow catheter tubing, which drains the CSF from the lateral ventricle; (2) a one-way pressure cutoff valve which regulates the intracranial pressure by controlling the amount of fluid which flows through the tubing; and (3) an outflow (distal) catheter at the other end of the tubing, which is located lower in the dog's body, such as in the abdomen, to be absorbed into the bloodstream and passed through the urinary system. The catheters and tubing usually are made of radiopaque silicone.
A computed tomography (CT) view showing
location of ventriculoperitoneal shunt placement, from its insertion
into the skull and down into the abdomen. Arrow points to the one-way
pressure cut-off valve.
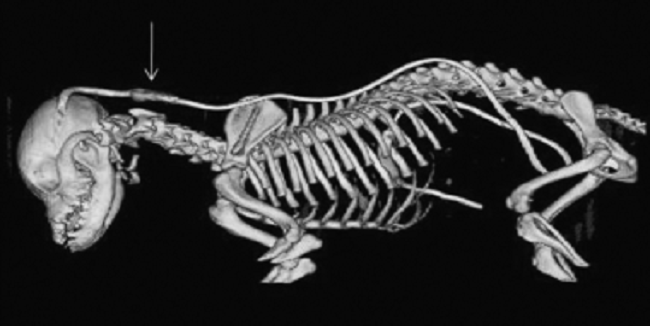
VP shunts have become a standard method of treating hydrocephalus in dogs. At the 2023 meeting of the British Veterinary Neurology Society, Dr. Ana Fernandez Cid reported on the successful insertion of a VPS for management of CM/SM in a cavalier. The series of MRI images below, courtesy of Dr. Rusbridge, show in (a) the arrow pointing to the enlarged ventricles, and in (b) a massive syrinx and developing pre-syrinx, prior to VP shunt surgery. In the post-operative MRI a month later, image (c) shows the two arrows pointing to the reduced size ventricles and the shunt within them. In (d), the arrow points to the completely collapsed syrinx and an almost normal spinal cord.
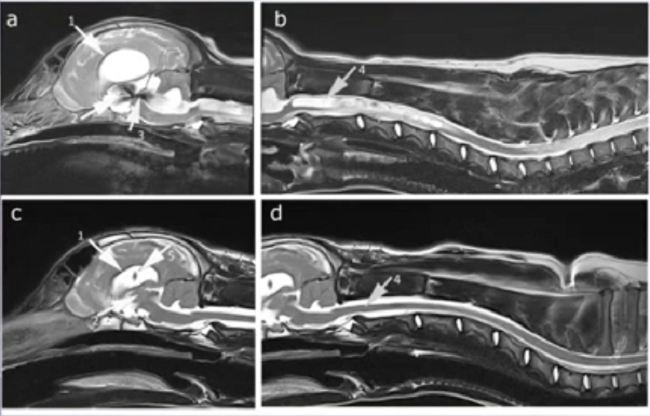
There are risks of compications and failure of VP shunts (e.g., see this April 2011 article), including subdural hemorrhage.
RETURN TO TOP
• Conclusion about surgeries
Dr. Rusbridge provides a detailed discussion of surgery on her YouTube channel at this link. She reports finding that:
• Surgery is an option particularly if the SM is progressive or is poorly controlled by medication.
• Shunts aer most likely to result in syrinx collapse but can be associated with serious complications.
• Foramen magnum decompression (FMD) does not seen to reverse the filling mechanism of the syrinx.
• FMD may result in improvement of symptoms.
Many of these studies have been "case studies", meaning that they were practiced without the controls normally included in clinical trials. In the July 2007 issue of Veterinary Surgery, Dr. Richard A. LeCouteur, board certified veterinary neurologist at the University of California, writes that:
"Medical history is replete with examples of invasive procedures and pharmacologic interventions that were widely accepted based on results of case studies, only to later be rejected based on results of controlled clinical trials. ... It's time to adopt a more structured scientific approach to the study of the management of neurologic conditions that may benefit from surgical intervention. The randomized (preferably) double-blinded (preferably) placebo-controlled study is the gold standard for evaluating a new treatment intervention."
RETURN TO TOP
Post-surgery soundwave therapy
 Some cavalier King Charles spaniels, which have continued to suffer
severe pain due to post-decompression surgery scar tissue, have been
very successfully treated with an infrasonic instrument called AlphaSonic™.
This infrasound technology generates multiple, random, chaotic sound waves
in the range of Alpha (approximately 8 to 14 Hz), and unlike ultrasound
waves, does not heat body tissue. Ultrasound uses a single high
frequency (from 20,000 to1,000,000 Hz) to stimulate a localized area and
heats tissue.
Some cavalier King Charles spaniels, which have continued to suffer
severe pain due to post-decompression surgery scar tissue, have been
very successfully treated with an infrasonic instrument called AlphaSonic™.
This infrasound technology generates multiple, random, chaotic sound waves
in the range of Alpha (approximately 8 to 14 Hz), and unlike ultrasound
waves, does not heat body tissue. Ultrasound uses a single high
frequency (from 20,000 to1,000,000 Hz) to stimulate a localized area and
heats tissue.
The manufacturer of the device represents that AlphaSonic™ is safer and more effective than ultrasound, penetrates deeper into the tissues, reduces inflammation, and softens scar tissue. It can be applied locally and at acupressure points, and is said to increase blood circulation and can allow the body to heal itself, much like the affects of acupuncture, but without the needles.
The device is electrically operated and looks very similar to an ultrasound unit. Dr. Ronald J. Riegel, DVM, who has studied the effects of the AlphaSonic™ since 2001, stated, "The goal of any physical therapy modality is to increase the circulation and increase the elasticity and flexibility of the tissue. the alphasonic absolutely increases circulation and allows the body to heal itself. The metabolism is increased, reducing recovery times".
Adequate hydration is important for optimum bodily function. The dog should be kept hydrated before, during, and after treatment with fresh clean water. Although the manufacturer reports that AlphaSonic™ is totally safe and that no negative side effects are known, any AlphaSonic™ treatments for dogs with veterinary conditions, especially those taking medication, should be performed only under the guidance of a qualified, licensed veterinarian. For more information about AlphaSonic™, contact Susan Stoltz at AlphaSonic, P.O. Box 2727, Valley Center, CA 92082, telephone 866-478-7118, email info@thealphasonic.com, website www.thealphasonic.com.
RETURN TO TOP
 Breeders' Responsibilities
Breeders' Responsibilities
SM is an inherited disorder that has a tendency to be more severe in each subsequent generation, and with an earlier onset. Breeders should follow the SM Breeding Protocol. Also, the confirmation indicator research described above is to aid in risk assessment to provide breeders with a tool to use with their breeding stock.
RETURN TO TOP
What You Can Do
• Send MRI scans of cavaliers 5 years old or older and which do not have SM, along with MRIs of those dogs' family members, to Dr. Clare Rusbridge at c.rusbridge@surrey.ac.uk.
• When lifting the dog, take care to support its entire body, including its head and neck. See this YouTube video as an example of how to lift the dog.
• Avoid "triggers" which can prompt a CM and/or SM reaction in the dog. The main trigger to avoid is walking the dog on a leash. Off leash exercise is preferable.
• Being professionally groomed can be extremely distressing for CM/SM-affected dogs, because the hands-on contact is constantly triggering.
 • Ease
your dog's symptoms by using a comfortable harness instead of a collar
attached to a
leash. The neck is one of the most vulnerable regions of the dog's
body. It houses the spinal cord, vertebrae, muscles, the
tongue bone, the thyroid gland, the trachea, the esosphagus, major
blood vessels, lymph nodes, and the thymus. Pulling on the collar
can permanently damage any or all of these vital features.
• Ease
your dog's symptoms by using a comfortable harness instead of a collar
attached to a
leash. The neck is one of the most vulnerable regions of the dog's
body. It houses the spinal cord, vertebrae, muscles, the
tongue bone, the thyroid gland, the trachea, the esosphagus, major
blood vessels, lymph nodes, and the thymus. Pulling on the collar
can permanently damage any or all of these vital features.
One of the best harnesses for cavaliers with CM/SM symptoms is the BRILLIANT K9 "Lucy Small" harness. It is easy to put on and easy to take off. Watch the videos: "Opening the harness" and "Walking the dog with the harness".
Provide affected dogs with raised food bowls so that they do not have to use their necks to lower their heads to reach their meals.
RETURN TO TOP
Research News
RETURN TO TOP
Related Links
CM and SM in Other Breeds
Other breeds known to be affected by Chiari-like malformation and
syringomyelia include the
Affenpinscher, Bichon Frise,
Boston terrier,
Brussels Griffon (Griffon Bruxellois), bull terrier,
Chihuahua, French
bulldog,
 Havanese, King Charles spaniel (the English toy spaniel),
Maltese
terrier, miniature dachshund, miniature and toy poodles,
Papillon,
Pomeranian,
Pug, Shih Tzu,
Staffordshire bull terrier, and the Yorkshire
terrier, as well as cross-breed dogs, particularly CKCS crosses. Up to
65% of MRI'd Griffon Bruxellois breed have been found to have CM. In a
September 2017 article, 100% of 53 Chihuahuas had CM and 38% of them had
SM. Click on the breeds' hyperlinked names to link to Internet articles
about CM and SM in those breeds. In a
December 2023 article, a study of 3,796 Pomeranians, 25% of them were
diagnosed with SM.
Havanese, King Charles spaniel (the English toy spaniel),
Maltese
terrier, miniature dachshund, miniature and toy poodles,
Papillon,
Pomeranian,
Pug, Shih Tzu,
Staffordshire bull terrier, and the Yorkshire
terrier, as well as cross-breed dogs, particularly CKCS crosses. Up to
65% of MRI'd Griffon Bruxellois breed have been found to have CM. In a
September 2017 article, 100% of 53 Chihuahuas had CM and 38% of them had
SM. Click on the breeds' hyperlinked names to link to Internet articles
about CM and SM in those breeds. In a
December 2023 article, a study of 3,796 Pomeranians, 25% of them were
diagnosed with SM.
In this October 2024 article, Dr. Paul Mandigers and fellow researchers studied MRIs of several small breeds, including the chihuahua, daschshund, French bulldog, Maltese, miniature schnauzer, Pekinese, pug, Shi Tzu, Yorkshire terrier, and a few un-named "various breeds/crosses". They report finding:
"There was no statistically significant difference for breed and sex (p = 0.32) or breed and age (p = 0.318), meaning that sex and age were comparable for all breeds. However, there was a statistically significant difference for all breeds for CM (p < 0.001), SM (p = 0.003) and syrinx size (p = 0.003), meaning that CM1/CM2 and SM+ were present at a significantly higher rate in all breeds. CM was seen the most in the Chihuahua (75%), Griffon (96%), Maltese (83%), Pug (75%) and Yorkshire terrier (80%) breeds. CM and/or SM were only seen in one out of nine Dachshunds. SM was observed in all breeds, with the highest frequency in the Griffon (89%), French Bulldog (70%) and Chihuahua (62%). Only in the French Bulldog was there evidence of a high number of dogs being affected by SM and not CM. In all other breeds, CM and SM appeared to predominantly occur simultaneously. In all breeds, SM was observed with an average syrinx size of 2.9 ± 2.4 mm (Mean ± SD), with the French Bulldog, Pug, Griffon and Chihuahua having larger syrinxes. MEE was only seen in the French Bulldog, two Chihuahuas and four crosses.
* * *
"In this group of small dog breeds, scanned because of the presence of neurological signs, CM and SM occurred in all breeds. It must be stressed that this is not a randomly chosen group of dogs, and all dogs included were scanned for a diagnostic reason. ... But in this group of small dogs, CM and SM was seen in all breeds. For the brachycephalic breeds, this was expected. There is an association of CM and brachycephaly. The findings in the four typical brachycephalic breeds, the French bulldog, Chihuahua, Griffon, and Pug, are therefore not surprising. The presence of CM and SM in both the Chihuahua and Griffon has also been reported earlier. Reports on CM/SM in the French Bulldog or Pug are anecdotal. In 2012, the French Bulldog was actually used as a control group for a CKCS study but based on our findings, the French Bulldog is not a suitable control group for the CKCS. It appears to be one of the breeds with a high incidence of SM. And although the number of Pugs in this study was small, CM and/or SM appears to occur with similar frequencies as within the French bulldog."



CONNECT WITH US