Palmitoylethanolamide (PEA) and
related amides and the
cavalier King Charles spaniel
-
 Palmitoylethanolamide
(PEA)
Palmitoylethanolamide
(PEA) - Different Forms of PEA
- Treated Disorders
- Dosages of PEA
- Conflicts of Interest
- Related Amides
- Research News
- Related Links
- Veterinary Resources
Palmitoylethanolamide (PEA)
Palmitoylethanolamide (PEA) is a N-acylethanolamine molecule in a family of long-chain fatty acid amides called ALIAmides (autacoid local injury antagonism mechanism). Other ALIAmides include Adelmidrol (ADM) and palmitoyl-glucosamine (PGA).
PEA is present throughout the bodies of most animals and many plants and appears to be produced as needed -- "on demand" -- in response to damage due to certain types of injuries. Dogs have been found to produce PEA in the heart's myocardium in response to ischemic injuries, such as strokes. The dog's brain has been shown to have the capability to produce PEA. PEA levels in dogs, humans, and rats appear to rise or fall in response to cell damage, inflammation, and pain.
Synthetic versions of those other ALIAmides -- Adelmidrol (ADM) and palmitoylglucosamine (PGA) -- also have been found to serve to increase levels of PEA in dogs. Optimistic researchers are of the opinion that in cases of decreased levels of internally-produced (endogenous) levels of PEA, that external (exogenous) administration of synthetic PEA may "top off" the body's lessened supply.
Synthetic PEA is a product of
normal fatty acid synthesis from palmitic acid. It is found in many
common foods, particularly palm oil, oils of cottonseed, corn, peanut,
safflower and olive, as well as beef tallow, butter fat, cocoa butter,
and egg yolks.
The commercial version is most commonly
![]() manufactured from palm oil*.
manufactured from palm oil*.
See, also, this YouTube video about PEA by veterinary neurologist Dr. Clare Rusbridge.
* Palm oil: The palm oil cultivation industry has been destroying rainforests in Sumatra and Borneo in Indonesia and Malaysia, the only habitats of orangutans. If you are going to obtain PEA, we suggest that you do so only from vendors whose PEA has been manufactured with palm oil from sustainable sources and not the deforestation of rainforests. Read more about finidng sources of sustainable palm oil at www.orangutanlandtrust.com. To avoid palm oil as the source of PEA, read the ingredients descriptions on the brands of PEA in which you are interested.
RETURN TO TOP
Different Forms of PEA
Not all PEA is alike. There are at least 4 types of PEA, and the differences of those 4 are described below here. The differences are mainly the sizes of the particles. The larger the size of particle, the less soluble and less bioavailable. Micronization reduces the size of the particles of PEA. In short, since the basic (naïve) PEA is almost totally insoluble in water and therefore has very poor bioavailability, researchers use micronized or ultra-micronized PEA or water-dispersible PEA in their published studies.
• Basic PEA, called "naïve PEA", is almost totally insoluble in water and under gastrointestinal conditions and therefore the oral intake of it (rather than being injected directly into the abdomen) has very poor bioavailability, meaning that it does not get absorbed well in the dog's gut. See this May 2021 article and this July 2025 article.
• Micronized PEA (m-PEA or micro-palmitoylethanolamide) is a patented technique that reduces the diameter of PEA particles, making them absorbable in the intestine, which has been found to be more effective than ordinary basic PEA in activating PEA levels in blood plasma in dogs. See this August 2014 article.
• Ultra-micronized PEA (um-PEA), also patented, reduces the PEA particle size further, to enable it to cross the blood-brain barrier, likewise has been found to be much more effective than basic PEA. See this August 2014 article.
• Water-Dispersible PEA (PEA-WD), also patented, reduces the PEA to a powder which can be dispersed in cold water. It has been found to be 16 times more effective than basic PEA. See this July 2025 article.
• Hybrid versions of PEA: Additonally, PEA has been combined with other ingredients and used in some published studies. These include FenuMat-PEA (P-fen), which is a PEA hybrid combined with the herb fenugreek (trigonella foenum-graecum), and hybrids combined with resveratrol, quercetin, fisstin, daidzein, genistein, and boswellic acid. See this September 2024 article and this May 2025 article and this July 2025 article.
In an August 2014 study comparing the 3 versions of PEA -- basic, micronized, and ultra-micronized -- in treating rats for inflammatory pain, the researchers reported that the pain "parameters were significantly decreased by oral treatment with micronized PEA-m and ultramicronized PEA-um at each time point compared to nonmicronized PeaPure."
In a July 2025 article. the researchers compared the dissolution rate and bioavailability of basic PEA and water-dispersible PEA (PEA-WD )and reported finding that PEA-WD was 16 times more effective than basic PEA.
PEA micronization and ultra-micronization are patented (by Italian
company, EPITECH
Group SpA) processing techniques that reduce the diameter of the PEA
particle to a micronized or ultra-micronized size
 which optimizes the
PEA's absorbability along the intestine. Micronization increases the
drug's surface area, thereby improving its dissolution rate and
minimizing its absorption difficulties. The ultra-micronized size
also enables
the PEA to cross the blood-brain barrier. See this
February 2021
article.
which optimizes the
PEA's absorbability along the intestine. Micronization increases the
drug's surface area, thereby improving its dissolution rate and
minimizing its absorption difficulties. The ultra-micronized size
also enables
the PEA to cross the blood-brain barrier. See this
February 2021
article.
If a PEA product is not advertised as being micronized or ultra-micronized (or water-dispersible), then Dr. Clare Rusbridge advises that "You probably are wasting your money." A variety of brands of micronized and ultra-micronized PEA are offered on-line.

RETURN TO TOP
Treated Disorders
Canine research into the usefulness of PEA and its amide partners, adelmidrol (ADM) and palmitoylglucosamine (PGA), is scarce. Most studies focused upon rodents, and some upon humans, and in some such cases, to obtain more favorable results, the PEA has been injected into the abdomen rather than consumed orally. The studies tend to include low numbers of participants and are laden with conflicts of interest (see below) due to PEA patent-holders and vendors underwriting them. Even in a review article by consultants for one of the two patent holders and manufacturers of m-PEA and um- PEA, they concede that "clinical studies in veterinary patients are warranted".
Apart from the disorders listed above and discussed in some detail below, claims by PEA vendors have been made about using PEA to treat such canine disorders as dental pain, neuropathic pain, urinary tract disease, inflammatory and auto-immune disorders, and pulmonary fibrosis. We are not aware of any published truly scientific, clinical studies regarding PEA successfully treating such disorders in dogs. Even in rodent and human studies, the investigators suggest at the most, that PEA may be shown to be useful as an "add-on" therapy, to be used in addition to conventional drugs, such as gabapentin for neuropathic pain.
A significant point to keep in mind is that,while PEA is most talked about and commercially emphasized, the two other amides, adelmidrol (ADM) and palmitoyl-glucosamine (PGA), have been shown to be more successful than PEA in treating certain disorders, such as ADM as a topical ointment for treating atopic dermatitis.
Atopic Dermatitis
See more about atopic dermatitis on our Skin Conditions webpage.
Dogs affected by atopic dermatitis (AD) have been found to have significantly elevated levels of their self-produced PEA, when compared to dogs without AD. This indicates the "on demand" aspect of PEA, that the dog produces more of its own PEA when needed to combat inflammation and other causes of skin eruptions. See this January 2014 article.
In an August 2015 article sponsored by the major supplier of PEA (Innovet Italia), Italian researchers conducted an 8-week study of the effectiveness of oral ultra-micronized palmitoylethanolamide (um-PEA) in 160 dogs with moderate atopic dermatitis. Each dog received a daily dose of um-PEA at the rate of 10 mg/kg for 56 days. The investigators concede some major limitations in the study but conclude that the number of participating dogs -- 160 -- offsets those limitations. They concluded:
"Notwithstanding the above limitations, our findings suggest that PEA-um, within a multifaceted and integrated therapeutic approach for cAD [canine atopic dermatitis], may be a promising treatment for dogs with moderate atopic dermatitis and moderate pruritus, with consequent improvement in QoL [quality of life]."
By "multifaceted and integrated therapeutic approach", they meant that the um-PEA was given in combination with more conventional AD medications.
Adelmidrol has been found to induce an increase in the natural PEA concentrations in the keratinocyte cells of canine skin. In a January 2016 study of canine keratinocytes cells, the predominant cell in dogs' skin (epidermis), the researchers found that adelmidrol increased PEA concentrations in canine keratinocytes and in the other cellular systems studied. They stated that their study demonstrates for the first time an ‘entourage effect’ of adelmidrol on PEA concentrations in keratinocytes. they conlcuded:
"These data substantiate the use of adelmidrol-containing topical preparations (currently available in the human and veterinary market) as adjuncts in the treatment of allergic-inflammatory disorders, especially of dermatologic nature."
Be aware that this study involved canine cells removed from the dogs and examined in vitro, as in a test tube.
In a May 2025 article, Indian researchers Harsh J Shah, Jacky K. Pariyani, Kalyani V. Shinde, Bhakti A. Dave, and Priyal B. Dakhore report treating 18 Labrador retrievers diagnosed with canine atopic dermatitis (CAD) with a combination of palmitoylethanolamide (PEA) and daidzein and genistein for 28 days. Daidzein and genistein are legume-based compounds called isoflavones, most commonly derived from soybeans. The article did not specify the form of PEA used in the study, although the authors referenced two other studies in which ultra-micronized PEA was the tested form.The researchers used the canine atopic dermatitis extent and severity index-4 (CADESI-4) to score the dermatitis in terms of severity and extent. The CADESI-4 was designed to comprise 20 body sites and three lesions (Erythema, Lichenification and Excoriations and/or Alopecia). There were four severity grades starting from 0=none, 1=mild, 2=moderate and 3=severe. Each site and each lesion type was given a score from 0 to 3 depending on the severity and extent of the dermatitis. Considering three lesions and four severity grades, a maximal score of 20 x 3 x 3=180 could be given. The researchers found that the PEA-combinati0n tabket resulted in the CADESI-4 scoring reduced gradually, and at the end of the study, the dogs' dermatitis condition has improved, both in extent and serverity. Also, the dogs' quality of life improved significantly after the 28 days study. The study was funded by Enavant Research LLP, a producer of PEA in India, and all of the researchers were employees of that company.
RETURN TO TOP
Dental Gingivitis
See more about dental disorders on our Dental Disorders (Periodontal Disease) webpage.
There are no published studies in which PEA has been given to treat or prevent dental and gum disorders. However, in a March 2008 article, adelmidrol has been successfully used to reduce inflammation in the mouths of 10 dogs diagnosed with gingivitis. Twenty dogs had undergone a dental prophylaxis, including a dental scaling, to treat their periodontal disease. Following the dentals, half of the 20 then were treated for 45 days with an oral gel which included adelmidrol as its active ingredient. The researchers compared the two groups' gingivitis index measurements at the 30 and 45 day marks and found that the adelmidrol dogs' level of gingival inflammation had regressed significantly, compared to the control group.
RETURN TO TOP
Osteoarthritis
See more about osteoarthritis on our Arthritis webpage.
There are no published veterinary journal articles about treating canines with PEA for osteoarthritis (OA). This is very limited research regarding a combination of ultramicronized PEA (um-PEA) and quercetin (PEA-Q), first in rats and finally in dogs.
The rat research has been an August 2017 article. The authors report that a combination of ultramicronized PEA and quercetin (PEA-Q), given orally to 10 rats. They reported:
"PEA-Q is a novel co-ultramicronized formulation of PEA and quercetin whose effects were investigated in two pre-clinical models of OA pain in rats. Oral administration of PEA-Q decreased pain sensitivity, improved locomotor function, reduced inflammatory signs and mediators and lowered histological damage score."
In that article, the authors go to great lengths to suggest that their "collective observations presented here propose that PEA-Q shows promise for multimodal pain management in canine and feline OA."
In a September 2018 report, 13 dogs diagnosed with chronic OA and lameness received supplements of PEA-Q for four weeks, at the rate of 24 mg/kg per day. Pain scores were taken, showing successful improvement in 7 of the dogs by the second week. Lameness also reportedly improved during the treatment period.
Nevertheless, at least one PEA vendor advertises that PEA alone (meaning, not even ultramicronized PEA and not combined with quercetin) "may be beneficial for ... Osteoarthritis pain". So, Buyer Beware. See more about this type of hazard below.
PEA's fellow ALIAmide, micronized palmitoyl-glucosamine (m-PGA), has been teamed with curcumin (m-PGA-Cur) to treat dogs with osteoarthritis. In an October 2020 report of 181 dogs diagnosed with OA, they were given m-PGA-Cur for 2 months in addition to their conventional medications. The dogs were scored for lameness and pain at the start of the study and each 30 days thereafter, along with having the dogs' owners evaluate their dogs' mobility impairment and pain behaviors. At the end of the study, 74% of the owners scored their dogs' quality of life as improved, and 87% of the 27 participating veterinarians expressed satisfaction with the addition of m-PGA-Cur in the daily management of the OA. This was not a controlled clinical trial, however.
In a February 2023 article, m-PGA and curcumin again were teamed (m-PGA-Cur) to treat 58 dogs having chronic OA pain. All of the dogs had been treated with meloxicam and continued to do so during the 18-week testing period. The aim of the study was to determine if adding m-PGA-Cur to the treatment regime enabled the meloxicam to be tapered at the rate of 25% during the first 8 weeks of combined treatment without experiencing worsening of pain. The investigators found that 75% of the dogs were assessed as having no pain increases 10 weeks after the withdrawal of meloxicam. They concluded that the m-PGA-Cur was able to maintain the level of pain relief previously maintained only by the meloxicam.
RETURN TO TOP
Syringomyelia
See more about syringomyelia on our Syringomyelia webpage.
There is no published clinical research supporting PEA to treat syringomyelia. Veterinary neurologist Dr. Clare Rusbridge spoke recently about the lack of any published studies regarding treating dogs for pain or neuro-degenerative diseases with PEA. She said:
“There are no clinical studies published as scientific articles. And yet, you can find an awful lot of YouTube articles and stuff on the Internet. And it bothers me that is often being pushed by people actually selling the drug. And they are using very unscientific parameters for assessing whether something is effective.”
While no objective, unbiased clinical studies of the effect of PEA on treating pain due to Chiari-like malformation (CM) and syringomyelia (SM) have been published, one under-powered pilot study, the April 2011 Normast Study, does exist, and it involved cavalier King Charles spaniels. Twelve cavaliers, all diagnosed with CM/SM by MRI-scans, were treated exclusively with micronized PEA for 2 months at a daily dose of 30 to 40 mg/kg. The owners of the dogs were asked to score signs of pain and improvement or lack thereof during the testing period. The owners' reports were necessarily subjective, but the summary of their reports is:
"More specifically: General impresison: in 4 dogs better and in 8 dogs much better. ; cheery/ lively: same 0, better: 5 much better: 7; headache (eyes closed): same 0, better: 5, much better: 7; lacrimation: same: 3, less: 3 much less: 6; chewing and swallowing, same: 3, less: 3 much less: 5 (1 not scored); scratching, licking, rubbing: same: 2, less: 5 much less: 5; moving around: same: 2, better: 2 much better: 7 (1 not scored) Qualitative statements of owners One owner states she can pat the dog again, and the dog played again with younger dogs. Some owner stated that the dog’s skull was less hot. One owner of a dog suffering from chronic ear inflammation (PSOM) could touch the dog's head and ears again. In one dog the owner stated that the lordosis vanished, one other dog showed affective behaviour and touching was again possible, etc, etc. All dog owners noticed impressive positive changes in behaviour after treatment with PEA. Follow-up: meanwhile many dogs are treated for many months, and most dogs are still very much stable."
RETURN TO TOP
Dosages of PEA
Daily dosages of micronized and ultra-micronized PEA given to dogs in published studies have varied from 10 mg/kg to 40 mg/kg. For a 20 lb. dog, that would mean a range of from 1/16 teaspoon to 1/8 tsp. per day.
In this July 2025 article, the researchers compared basic PEA, micronized PEA, ultra-micronized PEA, and water-dispersed PEA in rats, using these dosages:
• Basic PEA: 100.67 mg/kg orally
• Micronized PEA: 101.48 mg/kg orally
• Ultra-micronized PEA: 100.96 mg/kg orally
• Water-dispersed PEA: 117.23 mg/kg orally
RETURN TO TOP
Conflicts of Interest
 Most of the published research studies as to either or both safety
and effectiveness of PEA in treating dogs have included very few dogs. None of these studies rank above "pilot studies".
Most of the published research studies as to either or both safety
and effectiveness of PEA in treating dogs have included very few dogs. None of these studies rank above "pilot studies".
Furthermore, most all of them have been funded or otherwise sponsored by organizations having financial interests in PEA products. Indeed, Dr. Rusbridge spoke recently about the lack of any published studies regarding treating dogs for pain or neuro-degenerative diseases with PEA. She said:
“There are no clinical studies published as scientific articles. And yet, you can find an awful lot of YouTube articles and stuff on the Internet. And it bothers me that is often being pushed by people actually selling the drug. And they are using very unscientific parameters for assessing whether something is effective.”
As a result, success claims made in these studies and about them may not be reliable, or even factual. For example, one retail vendor of PEA repeatedly states in advertisements in social media that its product "has been proven to work in multiple clinical trials." In other cases, even some veterinarians who advertise PEA products for sale on their websites and through social media outlets make claims about the value of their PEA products to treat disorders for which not even a pilot study has been published.
When PEA vendors use the word "may", as in "PEA may be beneficial for ...", it means they have no objective scientific evidence that it is of any value whatsoever in dogs, but they hype it anyway in hopes you buy it.
 It is important for pet owners to be able to spot clear conflicts of
interest, such as social media mavens touting supplements which they are
selling. This especially is true when the vendor also is a veterinarian, and
the vendor is encouraging using the supplement instead of the medications
prescribed by the patients’ veterinary specialists.
It is important for pet owners to be able to spot clear conflicts of
interest, such as social media mavens touting supplements which they are
selling. This especially is true when the vendor also is a veterinarian, and
the vendor is encouraging using the supplement instead of the medications
prescribed by the patients’ veterinary specialists.
RETURN TO TOP
Related Amides
Adelmidrol (ADM)
Adelmidrol (ADM) is in the family of long-chain fatty acid amides called ALIAmides, it is a derivative of azelaic acid. It is known to have anit-inflammatory properties, similar to PEA, but has been found to be more effective as a topical application on the skin.
Adelmidrol also has been found to induce an increase in the natural PEA concentrations in the keratinocyte cells of canine skin.
Palmitoyl-glucosamine (PGA)
Palmitoyl-glucosamine (PGA) is also in the family of long-chain fatty acid amides called ALIAmides, it is an amide of palmitic acid and glucosamine. It is in the same family as PEA and in general, performs in the same fashion as does PEA, but it has been found in its micronized format to more successfully treat certain disorders, particularly osteoarthritis, than does PEA in the limited studies which have been published.
In a November 2019 article, PGA in micronized formulation (m-PGA) was determined to "greatly enhance" the activity of PGA and be superior to normal PGA in pain relief, anti-inflammation, and joint-protective effects in treating osteoarthritis in rats.
PGA typically is teamed with curcumin (m-PGA-Cur), the main active ingredient in turmeric (Curcuma longa L). Curcumin is known to have low water solubility and chemical instabiity, meaning it alone has very low bioavailability.
In an October 2020 report of 181 dogs diagnosed with OA, they were given m-PGA-Cur for 2 months in addition to their conventional medications. The dogs were scored for lameness and pain at the start of the study and each 30 days thereafter, along with having the dogs' owners evaluate their dogs' mobility impairment and pain behaviors. At the end of the study, 74% of the owners scored their dogs' quality of life as improved, and 87% of the 27 participating veterinarians expressed satisfaction with the addition of m-PGA-Cur in the daily management of the OA. This was not a controlled clinical trial, however.
In a February 2023 article, m-PGA and curcumin again were teamed (m-PGA-Cur) to treat 58 dogs having chronic OA pain. All of the dogs had been treated with meloxicam and continued to do so during the 18-week testing period. The aim of the study was to determine if adding m-PGA-Cur to the treatment regime enabled the meloxicam to be tapered at the rate of 25% during the first 8 weeks of combined treatment without experiencing worsening of pain. The investigators found that 75% of the dogs were assessed as having no pain increases 10 weeks after the withdrawal of meloxicam. They concluded that the m-PGA-Cur was able to maintain the level of pain relief previously maintained only by the meloxicam.
RETURN TO TOP
Research News
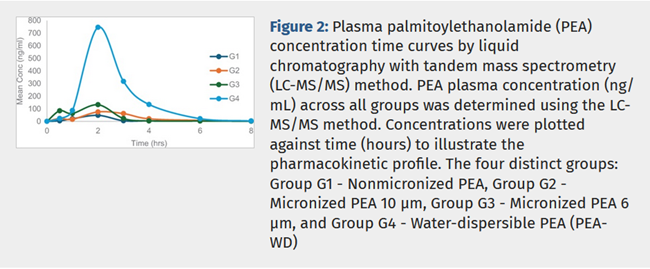
July 2025:
Water-dispersible PEA is found to be 16 times more bio-available
than basic, unmicronized PEA.
 In
a
July 2025 article, Indian researchers (S. Mehkri, K. G. Dinesh, G.
Ashok, Krathish Bopanna [right]) compared the dissolution and
absorption rates of single oral doses of the four types of
palmitoylethanolamide (PEA) -- basic PEA, micronized PEA,
ultra-micronized PEA, and water-dispersible PEA -- in groups of 6 rats
each over an 8 hour period. They report finding that the micronized and
ultra-micronized and water-dispersible PEA were far superior to the
basic PEA in improving the dissolution rate and bioavailability, and
that the water-dispersible PEA was 16 times more effective than basic
PEA. (See Figure 2, below. G2 is micronized, and G3 is
ultra-micromized.)
In
a
July 2025 article, Indian researchers (S. Mehkri, K. G. Dinesh, G.
Ashok, Krathish Bopanna [right]) compared the dissolution and
absorption rates of single oral doses of the four types of
palmitoylethanolamide (PEA) -- basic PEA, micronized PEA,
ultra-micronized PEA, and water-dispersible PEA -- in groups of 6 rats
each over an 8 hour period. They report finding that the micronized and
ultra-micronized and water-dispersible PEA were far superior to the
basic PEA in improving the dissolution rate and bioavailability, and
that the water-dispersible PEA was 16 times more effective than basic
PEA. (See Figure 2, below. G2 is micronized, and G3 is
ultra-micromized.)
May 2025: PEA combined with daidzein and genistein reduced dermatiis in 18 Labradors after 28 days. In a May 2025 article, Indian researchers Harsh J Shah, Jacky K. Pariyani, Kalyani V. Shinde, Bhakti A. Dave, and Priyal B. Dakhore report treating 18 Labrador retrievers diagnosed with canine atopic dermatitis (CAD) with a combination of palmitoylethanolamide (PEA) and daidzein and genistein for 28 days. Daidzein and genistein are legume-based compounds called isoflavones, most commonly derived from soybeans. The article did not specify the form of PEA used in the study, although the authors referenced two other studies in which ultra-micronized PEA was the tested form.The researchers used the canine atopic dermatitis extent and severity index-4 (CADESI-4) to score the dermatitis in terms of severity and extent. The CADESI-4 was designed to comprise 20 body sites and three lesions (Erythema, Lichenification and Excoriations and/or Alopecia). There were four severity grades starting from 0=none, 1=mild, 2=moderate and 3=severe. Each site and each lesion type was given a score from 0 to 3 depending on the severity and extent of the dermatitis. Considering three lesions and four severity grades, a maximal score of 20 x 3 x 3=180 could be given. The researchers found that the PEA-combinati0n tabket resulted in the CADESI-4 scoring reduced gradually, and at the end of the study, the dogs' dermatitis condition has improved, both in extent and serverity. Also, the dogs' quality of life improved significantly after the 28 days study. The study was funded by Enavant Research LLP, a producer of PEA in India, and all of the researchers were employees of that company.
February 2023:
Palmitoyl-glucosamine (m-PGA) and curcumin combine to relieve 58 dogs
with osteoarthritis.
 In
a
February 2023 article, a team of Italian researchers ( Giorgia della
Rocca [right], Carlo Schievano, Alessandra Di Salvo, Maria
Beatrice Conti, Maria Federica della Valle) treated 58 dogs having
chronic OA pain with m-PGA and curcumin (m-PGA-Cur) . All of the dogs
had been treated with meloxicam and continued to do so during the
18-week testing period. The aim of the study was to determine if adding
m-PGA-Cur to the treatment regime enabled the meloxicam to be tapered at
the rate of 25% during the first 8 weeks of combined treatment without
experiencing worsening of pain. The investigators found that 75% of the
dogs were assessed as having no pain increases 10 weeks after the
withdrawal of meloxicam. They concluded that the m-PGA-Cur was able to
maintain the level of pain relief previously maintained only by the
meloxicam.
In
a
February 2023 article, a team of Italian researchers ( Giorgia della
Rocca [right], Carlo Schievano, Alessandra Di Salvo, Maria
Beatrice Conti, Maria Federica della Valle) treated 58 dogs having
chronic OA pain with m-PGA and curcumin (m-PGA-Cur) . All of the dogs
had been treated with meloxicam and continued to do so during the
18-week testing period. The aim of the study was to determine if adding
m-PGA-Cur to the treatment regime enabled the meloxicam to be tapered at
the rate of 25% during the first 8 weeks of combined treatment without
experiencing worsening of pain. The investigators found that 75% of the
dogs were assessed as having no pain increases 10 weeks after the
withdrawal of meloxicam. They concluded that the m-PGA-Cur was able to
maintain the level of pain relief previously maintained only by the
meloxicam.
RETURN TO TOP
RETURN TO TOP
Veterinary Resources
Catabolism of N-Acylethanolamine Phospholipids by Dog Brain Preparations. V. Natarajan, P. C. Schmid, P. V. Reddy, H. H. 0. Schmid. J. Neurochemistry. January 1984; doi: 10.1111/j.1471-4159.1984.tb12750.x Quote: N-Acylphosphatidylethanolamine, incubated with dog brain homogenate or microsomes, was hydroyzed to phosphatidic acid and N-acylethanolamine by a phosphodiesterase of the phospholipase D type. In the absence of F−, phosphatidic acid was further hydrolyzed to diacylglycerol and Pi while N-acylethanolamine was hydrolyzed by an amidase to fatty acid and ethanolamine. The phosphodiesterase showed an alkaline pH optimum and was also active towards N-acetylphosphatidyletha-nolamine, N-acyl-lysophosphatidylethanolamine, and glycerophospho(N-acyl)ethanolamine but showed little activity toward phosphatidylethanolamine and phosphati-dylcholine. Ca2+ stimulated slightly at low concentrations but inhibited at higher concentrations. Triton X-100 stim ulated the hydrolysis of N-acylphosphatidylethanol-amine, inhibited that of N-acyl-lysophosphatidyletha-nolamine and glycerophospho(N-acyl)ethanolamine, and had no effect on phosphatidylethanolamine or phospha-tidylcholine hydrolysis. The N-acylethanolamine hydrolase (amidase) was also present in the microsomal fraction and exhibited a pH optimum of 10.0. In addition to hydrolysis by the phosphodiesterase, N-acylphosphati-dylethanolamine was also catabolized by microsomal phospholipases A1 and/or A2 to N-acyl-lysophosphati-dylethanolamine, some of which was further hydrolyzed to glycerophospho(N-acyl)ethanolamine.
Effect of a mucoadhesive gel and dental scaling on gingivitis in dogs. D Bonello, P Squarzoni. J. Vet. Dent. March 2008; doi: 10.1177/089875640802500108. Quote: Twenty client-owned dogs diagnosed with gingivitis were studied over a 45-day period in order to investigate the effect of professional dental prophylaxis combined with the use of a topical mucoadhesive gel containing adelmidrol, an aliamide. A non-intrusive papillary-marginal-gingival index (PMGI) was measured at each assessment, while the gingivitis index (GI) was measured only at the beginning and end of the study. Compared to the control group, the treated dogs had a significant decrease (P < 0.005) in the average GI index during the course of the study. A significant reduction (P < 0.002) in the average PMGI index was observed in both groups 15-days following dental prophylaxis. However at 30 and 45-days following dental prophylaxis, the PMGI index values were significantly different (P < 0.005) from baseline only in treated dogs. These results suggest that the combined use of a mucoadhesive gel with dental scaling was able to improve the regression of gingival inflammation and lengthen the therapeutic benefits of dental scaling and polishing during a limited study period.
Dynamic regulation of the endocannabinoid system: implications for analgesia. Devi Rani Sagar, A Gemma Gaw, Bright N Okine, Stephen G Woodhams, Amy Wong, David A Kendall, Victoria Chapman. Molecular Pain. October 2009; doi: 10.1186/1744-8069-5-59. Quote: The analgesic effects of cannabinoids are well documented, but these are often limited by psychoactive side-effects. Recent studies indicate that the endocannabinoid system is dynamic and altered under different pathological conditions, including pain states. Changes in this receptor system include altered expression of receptors, differential synthetic pathways for endocannabinoids are expressed by various cell types, multiple pathways of catabolism and the generation of biologically active metabolites, which may be engaged under different conditions. This review discusses the evidence that pain states alter the endocannabinoid receptor system at key sites involved in pain processing and how these changes may inform the development of cannabinoid-based analgesics.
Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFa release from canine skin mast cells. S. Cerrato, P. Brazis, M.F. della Valle, A. Miolo b, A. Puigdemont. Vet. Immunology & Immunopathology. June 2010; doi: 10.1016/j.vetimm.2009.06.011.Quote: Palmitoylethanolamide (PEA) is an endocannabinoid-like compound and the parent molecule of the aliamide family, a group of fatty acid amides able to act through the downregulation of mast cell degranulation. PEA has been proven to exert both analgesic and anti-inflammatory activity, and recent studies have shown its ability in reducing clinical symptoms of inflammatory skin diseases, both in humans and in animals. Although its pharmacological efficacy is well known, the mechanism of action of this family of compounds is still unclear. To better understand the cellular effects of aliamides in dogs, canine mast cells freshly isolated from skin biopsies were incubated with IgE-rich serum and were challenged with anti-canine IgE. Histamine, prostaglandin D2 (PGD2) and tumour necrosis factor-alpha (TNFa) release was measured in the presence and absence of increasing concentrations of PEA, ranging from 10-8M to 10-5 M. Histamine, PGD2 and TNFa release, immunologically induced by canine anti-IgE, were significantly inhibited in the presence of PEA. The maximuminhibitory effect on histamine release was observed at 3 x10-6 M PEA concentration achieving an inhibition of 54.3 ± 5.2%. PGD2 release was significantly inhibited at 10-5 M and 10-6 M PEA concentrations with 25.5±10.2% and 14.6± 5.6% of inhibition, respectively. Finally, PEA inhibited TNFa release to 29.2 ± 2.0% and 22.1 ± 7.2%, at concentrations of 10-5 M and 3 x 10-6 M, respectively. The results obtained in the present study showed the ability of the aliamide PEA to down-modulate skin mast cell activation. Therefore, our findings suggest that the beneficial effect of PEA, observed in inflammation and pain clinical studies, could be due, at least in part, to its ability to inhibit the release of both preformed and newly synthesised mast cell mediators.
Syringomyelia in Cavalier spaniels treated successfully with palmitoylethanolamide (PEA). Jan M. Keppel Hesselink. Instituut voor Neuropathische Pijn. April 2011. Quote: "In central neuropathic pain inflammatory and gial pathology might play a more important role than we previously thought. Recently in this field new drugtargets emerged and research into the treatment of central neuropathic pain is now slowly unfolding. One of those targets is the glia, and the phrase gliopathic pain has been used to point out the importance of the glia in neuropathic pain. The enodcannabinoid palmitoylethanolamide is a new analgesic compound within this new field. We conducted a pilot trial using palmitoylethanolamide in a animal model for central neuropathic pain, the cavalier KIng Charles spaniel, suffering from Arnold Chiari malformations and syringomyelia, and exposing classical pain behaviour. ... Central neuropathic pain is a prominent feature in 50 to 90% of adult human patients with syringomyelia. Recently, a direct relationship has been described between central neuropathic pain and objective markers of spinal cord damage. Large syrinxes leading to damage to the dorsal part of the spinal cord are leading to abnormal behaviour seen by cavalier King Charles spaniels. This behaviour is likely to be "neuropathic pain," and therefore it is suggested that conventional analgesic medication may be ineffective. ... In the Netherlands we conducted an open pilot trial and included 12 Cavalier king Charles spaniels, alle with MRI-scan positive syringomyelia and all suffering from behavioural abnormalities and objective signs related to syringomyelia and neuropathic pain. ... All dogs were weaned off their analgesic medication before entering the trial. During 2 months PEA was given in a dose of around 30 mg/kg BW. So most dogs received 150 mg twice daily. If dogs are very light (less then 6 kg) we start with PEA 200 mg. Dogs between 6-12 kg mostly get twice daily 150 mg and heavy dogs, and non responders twice daily 200 mg. As the substance isn tolerated very well, a bit more is no problem, up to 40 mg/kg dog. If dogs get too a higher dose, they start painting and they become restless. A composite scale scored by the dog owners was used, based on whether symptoms were the same, improved a bit or improved a lot. The first item was the overall general impression, the other items were linked to behavioural abnormalities, such as: cheery, lively (same, better, much better); headache (eyes closed) (same, better, much better); lacrimation (same, less, much less); chewing and swallowing, (same, less, much less); scratching, licking, rubbing (same, less, much less) ; moving around (same, better, much better). Results: After 2 months of PEA use in 12 Cavalier spaniels, the results were the following. Of all the 12 dogs, based on the overal general impression the owners could clearly detect improvement within 8 days, and in 5 dogs the improvement was seen already after 3-4 days. Within 4 weeks all dogs showed improvements. More specifically: General impresison: in 4 dogs better and in 8 dogs much better. ; cheery/ lively: same 0, better: 5 much better: 7; headache (eyes closed): same 0, better: 5, much better: 7; lacrimation: same: 3, less: 3 much less: 6; chewing and swallowing, same: 3, less: 3 much less: 5 (1 not scored); scratching, licking, rubbing: same: 2, less: 5 much less: 5; moving around: same: 2, better: 2 much better: 7 (1 not scored) Qualitative statements of owners One owner states she can pat the dog again, and the dog played again with younger dogs. Some owner stated that the dog’s skull was less hot. One owner of a dog suffering from chronic ear inflammation (PSOM) could tough the dogs head and ears again. In one dog the owner stated that the lordosis vanished, one other dog showed affective behaviour and touching was again possible, etc, etc. All dog owners noticed impressive positive changes in behaviour after treatment with PEA. Follow-up: meanwhile many dogs are treated for many months, and most dogs are still very much stable. The first signs of efficacy is less inflammation of the eyes, and less lacrimation, already sometimes after some days. As syringomyelia is a spinal cord condition, is it important to point out that palmitoylethanolamide has been evaluated in other models of spinal cord injury too. And these models supported its therapeutic role. Palmitoylethanolamide is the naturally occurring amide of palmitic acid and ethanolamine. In a great number of pharmacological trials this molecule has been shown to reduces pain and inflammation through the nuclear receptor PPAR-alpha. This receptor is activated by PEA. PEA can reduce secondary damage induced by experimental spinal cord injury (SCI) in a mice model. SCI leads to edema, neutrophil infiltration, and production of inflammatory mediators, tissue damage, and apoptosis. Repeated PEA administration significantly reduced: •1) the degree of spinal cord inflammation and tissue injury; •2) neutrophil infiltration; •3) nitrotyrosine formation; •4) proinflammatory cytokine expression; •5) nuclear transcription factor activation-kappaB activation; •6) inducible nitric-oxide synthase expression; and •6) apoptosis. Hand in hand with these biochemical improvements, PEA ameliorated the recovery of motor limb function. In a compression model induced by applying an aneurysm clip to the spinal cord in mice repeated PEA administration (10mg/kg i.p., 6 and 12h after SCI) significantly reduced the degree of the severity of spinal cord trauma through the reduction of mast cell infiltration and activation. PEA also significantly reduced the activation of microglia and astrocytes expressing cannabinoid CB(2) receptor after SCI. PEA also acted as a neuroprotectant via induction of neurotrophic factors. The results of this pilot trial are in line with experimental findings supporting the neuroprotective role of PEA, as well as its role as a anti-inflammatory and analgesic compound. The avaiibility of Normast [Peapure] for human and dog sufferers of central neuropathic pain is a leap forward in the treatment of this difficult to treat pain state."
Effects of palmitoylethanolamide on the cutaneous allergic inflammatory response in Ascaris hypersensitive Beagle dogs. Santiago Cerrato, Pilar Brazis, Maria Federica della Valle, Alda Miolo, Stefania Petrosino, Vincenzo Di Marzo, Anna Puigdemont. Vet. J. January 2012; doi: 10.1016/j.tvjl.2011.04.002. Quote: Palmitoylethanolamide (PEA) is an endogenous lipid mediator with anti-inflammatory and anti-hyperalgesic properties. The main objective of the present study was to evaluate the effects of PEA on the cutaneous allergic inflammatory reaction induced by different immunological and non-immunological stimuli in hypersensitive dogs. Six spontaneously Ascaris hypersensitive Beagle dogs were challenged with intradermal injections of Ascaris suum extract, substance P and anti-canine IgE, before and after a single oral administration of PEA at doses of 3, 10 and 30 mg/kg. A significant reduction in wheal area induced by both antigen and anti-canine IgE challenge was observed after PEA administration. No significant differences were observed between the two higher doses studied, suggesting that the 10 mg/kg dose had exerted the maximum inhibitory effect. When blood levels of PEA were compared with the effects at different times, an evident correlation was obtained. However, the anti-inflammatory effects of PEA were more long-lasting than their plasma concentrations. The intradermal injection of substance P did not reveal any skin reaction (wheal or erythema formation) at any of the concentrations tested. In conclusion, PEA might constitute a new therapeutic strategy for the treatment of allergic inflammatory skin diseases in companion animals.
Use of micronized palmitoylelalamide in the dog’s idiopathic gastrointestinal inflammation (IBD): description of 7 clinical cases. Pengo G, Miolo A. Proceedings 72nd International Congress SCIVAC, Milan, 23-25 March 2012. Paraphrase: PEA levels may increase in response to cell damage, as the colons of dogs with chronic enteropathy.
Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Stephen D. Skaper, Laura Facci, Mariella Fusco, Maria Federica della Valle, Morena Zusso, Barbara Costa, Pietro Giusti. Inflammopharmacol. November 2013; doi: 10.1007/s10787-013-0191-7. Quote: Persistent pain affects nearly half of all people seeking medical care in the US alone, and accounts for at least $80 billion worth of lost productivity each year. Among all types of chronic pain, neuropathic pain stands out: this is pain resulting from damage or disease of the somatosensory nervous system, and remains largely un- treatable. With few available treatment options, neuropathic pain represents an area of significant and growing unmet medical need. Current treatment of peripheral neuropathic pain involves several drug classes, including opioids, gabapentinoids, antidepressants, antiep- ileptic drugs, local anesthetics and capsaicin. Even so, less than half of patients achieve partial relief. This review discusses a novel approach to neuropathic pain manage- ment, based on knowledge of: the role of glia and mast cells in pain and neuroinflammation; the body’s innate mechanisms to maintain cellular homeostasis when faced with external stressors provoking, for example, inflamma- tion. The discovery that palmitoylethanolamide, a member of the N-acylethanolamine family which is produced from the lipid bilayer on-demand, is capable of exerting anti- allodynic and anti-hyperalgesic effects by down-modulat- ing both microglial and mast cell activity has led to the application of this fatty acid amide in several clinical studies of neuropathic pain, with beneficial outcome and no indication of adverse effects at pharmacological doses. Collectively, the findings presented here propose that pal- mitoylethanolamide merits further consideration as a disease-modifying agent for controlling inflammatory responses and related chronic and neuropathic pain.
Increased levels of palmitoylethanolamide and other bioactive lipid mediators and enhanced local mast cell proliferation in canine atopic dermatitis. Francesca Abramo, Luca Campora, Francesco Albanese, Maria Federica della Valle, Luigia Cristino, Stefania Petrosino, Vincenzo Di Marzo, Vincenzo Miragliotta.BMC Vet. Res. January 2014; doi: 10.1186/1746-6148-10-21. Quote: Background: Despite the precise pathogenesis of atopic dermatitis (AD) is unknown, an immune dysregulation that causes Th2-predominant inflammation and an intrinsic defect in skin barrier function are currently the two major hypotheses, according to the so-called outside-inside-outside model. Mast cells (MCs) are involved in AD both by releasing Th2 polarizing cytokines and generating pruritus symptoms through release of histamine and tryptase. A link between MCs and skin barrier defects was recently uncovered, with histamine being found to profoundly contribute to the skin barrier defects. Palmitoylethanolamide and related lipid mediators are endogenous bioactive compounds, considered to play a protective homeostatic role in many tissues: evidence collected so far shows that the anti-inflammatory effect of palmitoylethanolamide depends on the down-modulation of MC degranulation. Based on this background, the purpose of the present study was twofold: (a) to determine if the endogenous levels of palmitoylethanolamide and other bioactive lipid mediators are changed in the skin of AD dogs compared to healthy animals; (b) to examine if MC number is increased in the skin of AD dogs and, if so, whether it depends on MC in-situ proliferation. Results: The amount of lipid extract expressed as percent of biopsy tissue weight was significantly reduced in AD skin while the levels of all analyzed bioactive lipid mediators were significantly elevated, with palmitoylethanolamide showing the highest increase. In dogs with AD, the number of MCs was significantly increased in both the subepidermal and the perifollicular compartments and their granule content was significantly decreased in the latter. Also, in situ proliferation of MCs was documented. Conclusions: The levels of palmitoylethanolamide and other bioactive lipid mediators were shown to increase in AD skin compared to healthy samples, leading to the hypothesis that they may be part of the body’s innate mechanisms to maintain cellular homeostasis when faced with AD-related inflammation. In particular, the increase may be considered a temptative response to down-regulating the observed elevation in the number, functionality and proliferative state of MCs in the skin of AD dogs. Further studies are warranted to confirm the hypothesis.
Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. Daniela Impellizzeri, Giuseppe Bruschetta, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Emanuela Esposito, Salvatore Cuzzocrea. Neuroinflammation. August 2014; doi: 10.1186/s12974-014-0136-0. Quote: Background: The fatty acid amide palmitoylethanolamide (PEA) has been studied extensively for its anti-inflammatory and neuroprotective actions. The lipidic nature and large particle size of PEA in the native state may limit its solubility and bioavailability when given orally, however. Micronized formulations of a drug enhance its rate of dissolution and reduce variability of absorption when orally administered. The present study was thus designed to evaluate the oral anti-inflammatory efficacy of micronized/ultramicronized versus nonmicronized PEA formulations. Methods: Micronized/ultramicronized PEA was produced by the air-jet milling technique, and the various PEA preparations were subjected to physicochemical characterization to determine particle size distribution and purity. Each PEA formulation was then assessed for its anti-inflammatory effects when given orally in the carrageenan-induced rat paw model of inflammation, a well-established paradigm of edema formation and thermal hyperalgesia. Results: Intraplantar injection of carrageenan into the right hind paw led to a marked accumulation of infiltrating inflammatory cells and increased myeloperoxidase activity. Both parameters were significantly decreased by orally given micronized PEA (PEA-m; 10 mg/kg) or ultramicronized PEA (PEA-um; 10 mg/kg), but not nonmicronized PeaPure (10 mg/kg). Further, carrageenan-induced paw edema and thermal hyperalgesia were markedly and significantly reduced by oral treatment with micronized PEA-m and ultramicronized PEA-um at each time point compared to nonmicronized PeaPure. However, when given by the intraperitoneal route, all PEA formulations proved effective. Conclusions: These findings illustrate the superior anti-inflammatory action exerted by orally administered, micronized PEA-m and ultramicronized PEA-um, versus that of nonmicronized PeaPure, in the rat paw carrageenan model of inflammatory pain.
The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Stefania Petrosino, Aniello Schiano Moriello, Santiago Cerrato, Mariella Fusco, Anna Puigdemont, Luciano De Petrocellis, Vincenzo Di Marzo. Brit. J. Pharmacology. January 2015; doi: 10.1111/bph.13084. Quote: Background and Purpose: Palmitoylethanolamide (PEA) is an endogenous congener of anandamide and potentiates its actions at cannabinoid CB1 and CB2 receptors, and at transient receptor potential vanilloid type-1 (TRPV1) channels. The other endocannabinoid, 2-arachidonoylglycerol (2-AG), was recently suggested to act as a TRPV1 channel agonist. We investigated if PEA enhanced levels of 2-AG in vitro or in vivo and 2-AG activity at TRPV1 channels. Experimental Approach: Endogenous lipid levels were measured by LC-MS in (i) human keratinocytes incubated with PEA (10¨C20 |ÌM, 40 min, 6 and 24 h, 37¡ãC); (ii) the blood of spontaneously Ascaris suum hypersensitive beagle dogs given a single oral dose of ultramicronized PEA (30 mg¡¤kg.1, 1, 2, 4 and 8 h from administration); (iii) the blood of healthy volunteers given a single oral dose of micronized PEA (300 mg, 2, 4 and 6 h from administration). Effects of 2-AG at TRPV1 channels were assessed by measuring intracellular Ca2+ in HEK-293 cells over-expressing human TRPV1 channels. Key Results: PEA elevated 2-AG levels in keratinocytes (¡«3-fold) and in human and canine plasma (¡«2 and ¡«20-fold respectively). 2-AG dose-dependently raised intracellular Ca2+ in HEK-293-TRPV1 cells in a TRPV1-dependent manner and desensitized the cells to capsaicin. PEA only slightly enhanced 2-AG activation of TRPV1 channels, but significantly increased 2-AG-induced TRPV1 desensitization to capsaicin (IC50 from 0.75 ¡À 0.04 to 0.45 ¡À 0.02 |ÌM, with PEA 2 |ÌM). Conclusions and Implications: These observations may explain why several effects of PEA are attenuated by cannabinoid receptor or TRPV1 channel antagonists.
Protective effect of palmitoylethanolamide, a naturally-occurring molecule, in a rat model of cystitis. Federica Pessina , Raffaele Capasso , Francesca Borrelli , Teresa Aveta , Lorena Buono , Giuseppe Valacchi , Paolo Fiorenzani , Vincenzo Di Marzo , Pierangelo Orlando , Angelo A. Izzo. J. Urology. April 2015; doi: 10.1016/j.juro.2014.11.083. Quote: Purpose: PEA is an endogenous mediator released together with the endocannabinoid anandamide from membrane phospholipids. It is a plant derived compound with analgesic and anti-inflammatory properties. We verified whether the pathophysiology of experimental cystitis involves changes in the levels of PEA and of some of its targets, ie CB1 and CB2 receptors, and PPARα. We also determined whether exogenously administered PEA could be proposed as a preventive measure for cystitis. Materials and Methods: Cystitis was induced by cyclophosphamide in female rats. Nociceptive responses, voiding episodes, gross damage, myeloperoxidase activity, bladder weight, bladder PEA and endocannabinoid levels (measured by liquid chromatography-mass spectrometry) and the expression of PEA targets (measured by quantitative reverse transcriptase-polymerase chain reaction) were recorded. Results: Cyclophosphamide induced pain behavior, bladder inflammation and voiding dysfunction associated with increased bladder levels of PEA, up-regulation of CB1 receptor mRNA expression, down-regulation of PPARα mRNA and no change in CB2 receptor mRNA expression. Exogenously administered, ultramicronized PEA attenuated pain behavior, voids and bladder gross damage. The CB1 antagonist rimonabant and the PPARα antagonist GW6471 counteracted the beneficial effect of PEA on gross damage. Also, GW6471 further decreased voiding episodes in rats treated with PEA. Conclusions: The current study provides strong evidence for a protective role of PEA as well as an alteration in bladder levels of PEA and of some of its targets in cyclophosphamide induced cystitis.
Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: an open-label multi-centre study. Chiara Noli, M. Federica della Valle, Alda Miolo, Cristina Medori, Carlo Schievano. Vet. Dermatol. August 2015; doi: 10.1111/vde.12250. Quote: Background: Palmitoylethanolamide is a naturally occurring bioactive lipid, produced on-demand by damage-exposed cells. Palmitoylethanolamide is documented to counteract inflammation, itch and pain. Objective: The aim of this 8-week study was to evaluate the efficacy of oral ultra-micronized palmitoylethanolamide (PEA-um) in dogs with moderate atopic dermatitis. Animals: Clinicians from 39 veterinary clinics enrolled 160 dogs with nonseasonal atopic dermatitis and moderate pruritus. Methods: This was a multi-centre open-label study. On days 0 (D0) and 56 (D56), owners evaluated pruritus with a Visual Analog Scale (VAS) and completed a validated Quality of Life (QoL) questionnaire. Veterinarians assessed the severity of skin lesions using the Canine Atopic Dermatitis Lesion Index (CADLI). Results: Mean pruritus VAS score decreased from 5.7 ± 0.08 cm (range 3.8–7.9 cm) to 3.63 ± 0.19 cm (range 0.1–9.2 cm) (P < 0.0001). At D56, 58% of dogs showed a greater than 2 cm reduction from baseline and 30% showed an absent-to-very mild pruritus (VAS ≤ 2 cm). Mean total CADLI at D56 decreased significantly (P < 0.0001); in 62% of dogs this score reached a value in the remission range (≤5). Mean total QoL score was significantly decreased (P < 0.0001) with 45% of dogs reaching QoL values described for healthy animals. Tolerability was good-to-excellent with only four dogs reporting treatment associated reversible adverse events. Conclusions and clinical importance: PEA-um appears to be effective and safe in reducing pruritus and skin lesions, and in improving QoL in dogs with moderate atopic dermatitis and moderate pruritus.
Adelmidrol increases the endogenous concentrations of palmitoylethanolamide in canine keratinocytes and down-regulates an inflammatory reaction in an in vitro model of contact allergic dermatitis. S. Petrosino, A. Puigdemontc, M.F. della Valle, M. Fusco, R. Verde, M. Allarà, T. Aveta, P. Orlandof, V. Di Marzo. Vet. J. January 2016; doi: 10.1016/j.tvjl.2015.10.060. Quote: This study aimed to investigate potential newtarget(s)/mechanism(s) for the palmitoylethanolamide (PEA) analogue, adelmidrol, and its role in an in vitro model of contact allergic dermatitis. Freshly isolated canine keratinocytes, human keratinocyte (HaCaT) cells and human embryonic kidney (HEK)-293 cells, wildtype or transfected with cDNA encoding for N-acylethanolamine-hydrolysing acid amidase (NAAA),were treated with adelmidrol or azelaic acid, and the concentrations of endocannabinoids (anandamide and 2-arachidonoylglycerol) and related mediators (PEA and oleoylethanolamide)were measured. The mRNA expression of PEA catabolic enzymes (NAAAand fatty acid amide hydrolase, FAAH), and biosynthetic enzymes (N-acyl phosphatidylethanolamine-specific phospholipase D,NAPE-PLD) and glycerophosphodiester phosphodiesterase 1, was also measured. Brain or HEK-293 cell membrane fractions were used to assess the ability of adelmidrol to inhibit FAAH and NAAA activity, respectively. HaCaT cells were stimulated with polyinosinic–polycytidylic acid and the release of the pro-inflammatory chemokine, monocyte chemotactic protein-2 (MCP-2), was measured in the presence of adelmidrol. Adelmidrol increased PEA concentrations in canine keratinocytes and in the other cellular systems studied. It did not inhibit the activity of PEA catabolic enzymes, although it reduced their mRNA expression in some cell types. Adelmidrol modulated the expression of PEA biosynthetic enzyme, NAPE-PLD, in HaCaT cells, and inhibited the release of the pro-inflammatory chemokine MCP-2 from stimulated HaCaT cells. This study demonstrates for the first time an ‘entourage effect’ of adelmidrol on PEA concentrations in keratinocytes and suggests that this effect might mediate, at least in part, the anti-inflammatory effects of this compound in veterinary practice.
Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Linda Gabrielsson, Sofia Mattsson, Christopher J. Fowler. Brit. J. Clinical Pharmoacology. May 2016; doi: 10.1111/bcp.13020. Quote: Palmitoylethanolamide (PEA) has been suggested to have useful analgesic properties and to be devoid of unwanted effects. Here, we have examined critically this contention, and discussed available data concerning the pharmacokinetics of PEA and its formulation. Sixteen clinical trials, six case reports/pilot studies and a meta-analysis of PEA as an analgesic have been published in the literature. For treatment times up to 49 days, the current clinical data argue against serious adverse drug reactions (ADRs) at an incidence of 1/200 or greater. For treatment lasting more than 60 days, the number of patients is insufficient to rule out a frequency of ADRs of less than 1/100. The six published randomized clinical trials are of variable quality. Presentation of data without information on data spread and nonreporting of data at times other than the final measurement were among issues that were identified. Further, there are no head-to-head clinical comparisons of unmicronized vs. micronized formulations of PEA, and so evidence for superiority of one formulation over the other is currently lacking. Nevertheless, the available clinical data support the contention that PEA has analgesic actions and motivate further study of this compound, particularly with respect to head-to-head comparisons of unmicronized vs. micronized formulations of PEA and comparisons with currently recommended treatments.
The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Stefania Petrosino, Vincenzo Di Marzo. Brit. J. Pharmacology. January 2017; doi: 10.1111/bph.13580. Quote: Palmitoylethanolamide (PEA) has emerged as a potential nutraceutical, because this compound is naturally produced in many plant and animal food sources, as well as in cells and tissues of mammals, and endowed with important neuroprotective, antiinflammatory and analgesic actions. Several efforts have been made to identify the molecular mechanism of action of PEA and explain its multiple effects both in the central and the peripheral nervous system. Here, we provide an overview of the pharmacology, efficacy and safety of PEA in neurodegenerative disorders, pain perception and inflammatory diseases. The current knowledge of new formulations of PEA with smaller particle size (i.e. micronized and ultra-micronized) when given alone or in combination with antioxidant flavonoids (i.e. luteolin) and stilbenes (i.e. polydatin) is also reviewed.
Ultramicronized palmitoylethanolamide counteracts the effects of compound 48/80 in a canine skin organ culture model. Francesca Abramo, Giulia Lazzarini, Andrea Pirone, Carla Lenzi, Sonia Albertini, M. Frederica della Valle, Carlo Schievano, Iacopo Vannozzi, Vincenzo Miragliotta. Vet. Dermatology. June 2017; doi: 10.1111/vde.12456. Quote: Background – Ultramicronized palmitoylethanolamide (PEA-um) has been reported to reduce pruritus and skin lesions in dogs with moderate atopic dermatitis and pruritus. Hypothesis/Objectives – A canine ex vivo skin model was used to investigate the ability of PEA-um to counteract changes induced by compound 48/80, a well-known secretagogue that causes mast cell degranulation. Animals – Normal skin was obtained from three donor dogs subjected to surgery for reasons unrelated to the study. Methods – Cultured skin biopsy samples in triplicate were treated with 10 and 100 lg/mL compound 48/80, without or with 30 lM PEA-um. Mast cell (MC) degranulation, histamine release into the culture medium, local microvascular dilatation, epidermal thickness, keratinocyte proliferation and epidermal differentiation markers were evaluated. Results – Exposure of the skin organ culture to PEA-um 24 h before and 72 h concomitantly to compound 48/80 resulted in a significant decrease of degranulating MCs. PEA-um also reduced the histamine content in the culture medium by half, although the effect did not reach statistical significance. PEA-um significantly counteracted vasodilation induced by 100 lg/mL compound 48/80. Finally, PEA-um alone did not induce changes in epidermal thickness, differentiation markers, keratinocyte proliferation, MC density and/or degranulation. Conclusions and clinical importance – Collectively, these results support the protective action PEA-um on the skin of dogs undergoing allergic changes.
A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. Domenico Britti, Rosalia Crupi, Daniela Impellizzeri, Enrico Gugliandolo, Roberta Fusco, Carlo Schievano, Valeria Maria Morittu, Maurizio Evangelista, Rosanna Di Paola, Salvatore Cuzzocrea. BMC Vet. Res. August 2017; doi: 10.1186/s12917-017-1151-z. Quote: Osteoarthritis (OA) is a common progressive joint disease in dogs and cats. The goal of OA treatment is to reduce inflammation, minimize pain, and maintain joint function. Currently, non-steroidal anti-inflammatory drugs (e.g., meloxicam) are the cornerstone of treatment for OA pain, but side effects with long-term use pose important challenges to veterinary practitioners when dealing with OA pain. Palmitoylethanolamide (PEA) is a naturally-occurring fatty acid amide, locally produced on demand by tissues in response to stress. PEA endogenous levels change during inflammatory and painful conditions, including OA, i.e., they are typically increased during acute conditions and decreased in chronic inflammation. Systemic treatment with PEA has anti-inflammatory and pain-relieving effects in several disorders, yet data are lacking in OA. Here we tested a new composite, i.e., PEA co-ultramicronized with the natural antioxidant quercetin (PEA-Q), administered orally in two different rat models of inflammatory and OA pain, namely carrageenan paw oedema and sodium monoiodoacetate (MIA)-induced OA. Oral treatment with meloxicam was used as benchmark. Results: PEA-Q decreased inflammatory and hyperalgesic responses induced by carrageenan injection, as shown by: (i) paw oedema reduction, (ii) decreased severity in histological inflammatory score, (iii) reduced activity of myeloperoxidase, i.e., a marker of inflammatory cell infiltration, and (iv) decreased thermal hyperalgesia. Overall PEA-Q showed superior effects compared to meloxicam. In MIA-treated animals, PEA-Q exerted the following effects: (i) reduced mechanical allodynia and improved locomotor function, (ii) protected cartilage against MIA-induced histological damage, and (iii) counteracted the increased serum concentration of tumor necrosis factor alpha, interleukin 1 beta, metalloproteases 1, 3, 9 and nerve growth factor. The magnitude of these effects was comparable to, or even greater than, those of meloxicam. Conclusion: The present findings shed new light on some of the inflammatory and nociceptive pathways and mediators targeted by PEA-Q and confirm its anti-inflammatory and pain-relieving effects in rodent OA pain models. The translatability of these observations to canine and feline OA pain is currently under investigation.
Effect of Palmitoylethanolamide Co-Ultra Micronized with Quercetin in Dogs with Osteoarthritis by Means of Dynamic Gate Analysis and Canine Brief Pain Inventory Questionnaire. In Proceedings of the 5th World Veterinary Orthopaedic Congress ESVOT-VOS, Barcelona, Spain, 12–15 September 2018. Vezzoni, A.; Crupi, F.; Boiocchi, S.; Boano, S. September 2018; pp. 771–772. Paraphrase: an open-field trial performed in 13 medium-to-large-breed client- owned adult dogs, with chronic OA and persistent lameness longer than one month. All dogs were supplemented for 4 weeks with a complementary feed containing PEA co-ultramicronized with the natural antioxidant quercetin (i.e., PEA-q, 24 mg/kg body weight). The Canine Brief Pain Inventory (CBPI) questionnaire was used to assess the severity of chronic pain (PSS, Pain Severity Score) and how it interfered with the dog’s normal functioning (PIS, Pain Interference Score). With success defined as a reduction of ≥1 in PSS and PIS, treatment was classified as successful in 54.5% dogs as early as week 2 and CBPI scores significantly decreased throughout the study (Figure 7). Moreover, lameness (either scored by the veterinarian on a 0–4 clinical scale or objectively assessed through a dynamic gait analysis) was found to significantly .improve during the treatment period
Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Marika Cordaro, Rosalba Siracusa, Daniela Impellizzeri, Ramona D’ Amico, Alessio Filippo Peritore, Rosalia Crupi, Enrico Gugliandolo, Roberta Fusco, Rosanna Di Paola, Carlo Schievano, Salvatore Cuzzocrea. Arthritis Res. & Therapy. November 2019; doi: 10.1186/s13075-019-2048-y. Quote: Background: Osteoarthritis is increasingly recognized as the result of a complex interplay between inflammation, chrondrodegeneration, and pain. Joint mast cells are considered to play a key role in orchestrating this detrimental triad. ALIAmides down-modulate mast cells and more generally hyperactive cells. Here we investigated the safety and effectiveness of the ALIAmide N-palmitoyl-D-glucosamine (PGA) in inflammation and osteoarthritis pain. Methods: Acute toxicity of micronized PGA (m-PGA) was assessed in rats following OECD Guideline No.425. PGA and m-PGA (30 mg/kg and 100 mg/kg) were orally administered to carrageenan (CAR)-injected rats. Dexamethasone 0.1 mg/kg was used as reference. Paw edema and thermal hyperalgesia were measured up to 6 h post-injection, when also myeloperoxidase activity and histological inflammation score were assessed. Rats subjected to intra-articular injection of sodium monoiodoacetate (MIA) were treated three times per week for 21 days with PGA or m-PGA (30 mg/kg). Mechanical allodynia and motor function were evaluated at different post-injection time points. Joint histological and radiographic damage was scored, articular mast cells were counted, and macrophages were immunohistochemically investigated. Levels of TNF-α, IL-1β, NGF, and MMP- 1, MMP-3, and MMP-9 were measured in serum using commercial colorimetric ELISA kits. One- or two-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons was used. Results: Acute oral toxicity of m-PGA resulted in LD50 values in excess of 2000 mg/kg. A single oral administration of PGA and m-PGA significantly reduced CAR-induced inflammatory signs (edema, inflammatory infiltrate, and hyperalgesia), and m-PGA also reduced the histological score. Micronized PGA resulted in a superior activity to PGA on MIA-induced mechanical allodynia, locomotor disability, and histologic and radiographic damage. The MIA-induced increase in mast cell count and serum level of the investigated markers was also counteracted by PGA and to a significantly greater extent by m-PGA. Conclusions: The results of the present study showed that PGA is endorsed with anti-inflammatory, painrelieving, and joint-protective effects. Moreover, it proved that particle size reduction greatly enhances the activity of PGA, particularly on joint pain and disability. Given these results, m-PGA could be considered a valuable option in the management of osteoarthritis.
The Basal Pharmacology of Palmitoylethanolamide. Linda Rankin, Christopher J. Fowler. Intl. J. Molecular Sci. October 2020: doi: 10.3390/ijms21217942. Quote: Palmitoylethanolamide (PEA, N-hexadecanoylethanolamide) is an endogenous compound belonging to the family of N-acylethanolamines. PEA has anti-inflammatory and analgesic properties and is very well tolerated in humans. In the present article, the basal pharmacology of PEA is reviewed. In terms of its pharmacokinetic properties, most work has been undertaken upon designing formulations for its absorption and upon characterising the enzymes involved in its metabolism, but little is known about its bioavailability, tissue distribution, and excretion pathways. PEA exerts most of its biological e ects in the body secondary to the activation of peroxisome proliferator-activated receptor- (PPAR- ), but PPAR- -independent pathways involving other receptors (Transient Receptor Potential Vanilloid 1 (TRPV1), GPR55) have also been identified. Given the potential clinical utility of PEA, not least for the treatment of pain where there is a clear need for new well-tolerated drugs, we conclude that the gaps in our knowledge, in particular those relating to the pharmacokinetic properties of the compound, need to be filled.
Integrazione dietetica con PGA-Cur: indagine osservazionale su 181 cani con osteoartrite. (Dietary supplementation with PGA-Cur: a survey on 181 osteoarthritis dogs). Roberto Marco Asperio. Summa, Animali da Compagnia. October 2020;37(8):39-49. Quote: Osteoarthritis (OA) is the leading cause of lameness and chronic pain in dogs. Considering the current therapeutic landscape, there is room for new strategies to improve the management of OA, eventually combined with standard approaches. The aim of the present survey was to collect information on the addon use of a complementary dietetic feed containing PGA-Cur (co-micronized palmitoyl-glucosamine and curcumin) in the conservative management of OA dogs. The study product was included for two months in the standard management of OA, the latter consisting of one or more conservative measures, considered the most suitable in the opinion of the veterinary surgeon. Before administration and after 30 and 60 days, the veterinarian scored lameness and pain and asked the owner to evaluate dog mobility impairment and pain behaviours. A total of 27 veterinarians joined the survey and 181 dogs were included (62% older than 7 years). Most dogs suffered from secondary OA. The study dietetic feed showed beneficial effects on both owner and veterinarian-assessed outcome measures. 74% of owners scored their dog's quality of life as improved and 87% of veterinarians were satisfied with the outcome. The present real-life findings may pave the way for future controlled clinical trials aimed at confirming the usefulness of PGA-Cur in the multimodal management of canine OA.
Micronized / ultramicronized palmitoylethanolamide (PEA) as natural neuroprotector against COVID-19 inflammation. Luca Roncati, Beatrice Lusenti, Federica Pellati, Lorenzo Corsi. Prostaglandins & Other Lipid Mediators. February 2021; doi: 10.1016/j.prostaglandins.2021.106540. Quote: PEA micronization is a patented technique that allows to reduce PEA particle diameter up to a micronized size of 2 ± 6 μm (mPEA), optimally absorbable along the intestine, or to an ultramicronized size of 0.8 ± 2 μm (umPEA), able to cross the blood-brain barrier too.
Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Giorgia della Rocca, Davide Gamba. Animals. March 2021; doi: 10.3390/ani11040952. Quote: The management of chronic pain is an integral challenge of small animal veterinary practitioners. Multiple pharmacological agents are usually employed to treat maladaptive pain including opiates, non-steroidal anti-inflammatory drugs, anticonvulsants, antidepressants, and others. In order to limit adverse effects and tolerance development, they are often combined with non-pharmacologic measures such as acupuncture and dietary interventions. Accumulating evidence suggests that non-neuronal cells such as mast cells and microglia play active roles in the pathogenesis of maladaptive pain. Accordingly, these cells are currently viewed as potential new targets for managing chronic pain. Palmitoylethanolamide is an endocannabinoid-like compound found in several food sources and considered a body’s own analgesic. The receptor-dependent control of non-neuronal cells mediates the pain-relieving effect of palmitoylethanolamide. Accumulating evidence shows the anti-hyperalgesic effect of supplemented palmitoylethanolamide, especially in the micronized and co-micronized formulations (i.e., micro-palmitoylethanolamide), which allow for higher bioavailability. In the present paper, the role of non-neuronal cells in pain signaling is discussed and a large number of studies on the effect of palmitoylethanolamide in inflammatory and neuropathic chronic pain are reviewed. Overall, available evidence suggests that there is place for micro-palmitoylethanolamide in the dietary management of chronic pain in dogs and cats. ... Although clinical studies in veterinary patients are warranted, the reviewed findings lay the foundations for a scientific and rational use of micro-PEA in the dietary management of chronic pain in dogs and cats.
Palmitoylethanolamide: A Natural Compound for Health Management. Paul Clayton, Mariko Hill, Nathasha Bogoda, Silma Subah, Ruchitha Venkatesh. Int’l J.Molecular Sci. May 2021; doi: 10.3390/ijms22105305 Quote: All nations which have undergone a nutrition transition have experienced increased frequency and falling latency of chronic degenerative diseases, which are largely driven by chronic inflammatory stress. Dietary supplementation is a valid strategy to reduce the risk and severity of such disorders. Palmitoylethanolamide (PEA) is an endocannabinoid-like lipid mediator with extensively documented anti-inflammatory, analgesic, antimicrobial, immunomodulatory and neuroprotective effects. It is well tolerated and devoid of side effects in animals and humans. PEA’s actions on multiple molecular targets while modulating multiple inflammatory mediators provide therapeutic benefits in many applications, including immunity, brain health, allergy, pain modulation, joint health, sleep and recovery. ... PEA is lipophilic in nature and almost insoluble in water, and its poor solubility and bioavailability has limited the development of nutraceutical applications. ... PEA’s poor oral bioavailability, a major obstacle in early research, has been overcome by advanced delivery systems now licensed as food supplements. This review summarizes the functionality of PEA, supporting its use as an important dietary supplement for lifestyle management.
The effect of a mixed cannabidiol and cannabidiolic acid based oil on client-owned dogs with atopic dermatitis. Melissa Loewinger, Joseph J. Wakshlag, Daniel Bowden, Jeanine Peters-Kennedy, Andrew Rosenberg. Vet. Derm. May 2022; doi: 10.1111/vde.13077. Quote: Background: Cannabidiol (CBD) and cannabidiolic acid (CBDA) are reported to have antinociceptive, immunomodulatory and anti-inflammatory actions. Objectives: To determine if CBD/CBDA is an effective therapy for canine atopic dermatitis (cAD). Animals: Thirty-two privately owned dogs with cAD. Materials and methods: Prospective, randomised, double-blinded, placebo-controlled study. Concurrent therapies were allowed if remained unchanged. Dogs were randomly assigned to receive either 2 mg/kg of an equal mix of CBD/CBDA (n = 17) or placebo for 4 weeks. On Day (D)0, D14 and D28, Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI-04) and pruritus Visual Analog Scale (pVAS) scores were determined by investigators and owners, respectively. Complete blood count, serum biochemistry profiles and cytokine bioassays were performed on serum collected on D0 and D28. Results: Results of this study indicated that CBD/CBDA does not affect lesion severity yet does have a positive effect on pruritus as an adjunct therapy in some dogs with cAD. ...There was no significant difference in CADESI-04 from D0 to D14 or D28 in either group. pVAS scores were significantly lower for the treatment group at D14and D28 and a significant change in pVAS from baseline was seen at D14 and not D28 between groups. There was no significant difference in serum levels of interleukin (IL)-6, IL-8, monocyte chemoattractant protein - 1, IL-31 or IL-34 between groups at D0 or D28. Elevated alkaline phosphatase was observed in four of 17 treatment group dogs. Conclusions and clinical relevance: CBD/CBDA as an adjunct therapy decreased pruritus, and not skin lesions associated with cAD in dogs.
Palmitoylethanolamide and Related ALIAmides for Small Animal Health: State of the Art. Giorgia della Rocca, Giovanni Re. Biomolecules. August 2022; doi: 10.3390/ biom12091186. Quote: ALIAmides are a family of fatty acid amides whose name comes from their mechanism of action, i.e., the Autacoid Local Injury Antagonism (ALIA). Actually, the ALIAmide parent molecule, palmitoylethanolamide (PEA), is locally produced on demand from a cell membrane precursor in order to control immune-inflammatory cell responses, avert chronic non-resolving inflammation, and limit the resulting clinical signs. ALIAmide sister compounds, such as Adelmidrol and palmitoylglucosamine, share mechanisms of action with PEA and may also increase endogenous levels of PEA. Provided that their respective bioavailability is properly addressed (e.g., through decreasing the particle size through micronization), exogenously administered ALIAmides thus mimic or sustain the prohomeostatic functions of endogenous PEA. The aim of the present paper is to review the main findings on the use of ALIAmides in small animals as a tribute to the man of vision who first believed in this “according-to-nature” approach, namely Francesco della Valle. After briefly presenting some key issues on the molecular targets, metabolism, and pharmacokinetics of PEA and related ALIAmides, here we will focus on the preclinical and clinical studies performed in dogs and cats. Although more data are still needed, ALIAmides may represent a novel and promising approach to small animal health.
Palmitoyl‑glucosamine co‑micronized with curcumin for maintenance of meloxicam‑induced pain relief in dogs with osteoarthritis pain. Giorgia della Rocca, Carlo Schievano, Alessandra Di Salvo, Maria Beatrice Conti, Maria Federica della Valle. BMC Vet. Res. February 2023; doi: 10.1186/s12917-023-03594-4. Quote: Background: Osteoarthritis (OA) pain is the number one cause of chronic pain in dogs. Multimodal treatment, including combining safe and effective nutritional interventions with non-steroidal anti-inflammatory drugs (NSAIDs), is currently considered one of the most appropriate choices for managing OA pain. Palmitoyl-glucosamine is a feed material belonging to the ALIAmide family, whose parent molecule is the prohomeostatic lipid amide N-palmitoylethanolamine. Curcumin is a promising plant antioxidant. The present study aimed at investigating whether 18-week dietary integration with palmitoyl-glucosamine co-micronized with curcumin was able to maintain pain relief in dogs with OA-associated chronic pain receiving meloxicam (1.5 mg/ml oral suspension) on a tapering regimen (progressive 25% decrease of the original 0.1 mg/kg/day dose, on a biweekly basis) during the first 8 weeks of treatment. Pain was assessed both by the owners and veterinary surgeons, with the first using both subjective evaluation and validated metrology instruments—i.e., Helsinki Chronic Pain Index (HCPI) and Canine Brief Pain Inventory (CBPI)—while the second rating the severity of lameness and pain on palpation on two previously used 5-point scales. Results: A total of fifty-eight dogs with OA chronic pain entered the uncontrolled study. Pain on HCPI was considered severe at baseline (range 18–39). Based on owner’s assessment, 90% of dogs who responded to meloxicam at the full-dose regimen could reduce meloxicam up to 25% of the original dose without experiencing pain worsening. Moreover, 75% of dogs was assessed as having no pain increase ten weeks after meloxicam withdrawal. A statistically significant decrease of pain severity as scored by HCPI (P < 0.0001) was observed two and ten weeks after meloxicam withdrawal compared to study entry (17.0 ± 1.05 and 15.1 ± 1.02, respectively, vs 29.0 ± 0.74; mean ± SEM). After meloxicam withdrawal, no statistically significant change in the CBPI scores was recorded. Pain on palpation and lameness significantly changed to less severe distributions along the study period (P < 0.0001). Conclusion: The findings appear to suggest that dietary integration with palmitoyl-glucosamine co-micronized with curcumin was able to maintain meloxicam-induced pain relief in dogs with severe OA chronic pain.
Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Kordula Lang-Illievich, Christoph Klivinyi, Christian Lasser, Connor T. A. Brenna , Istvan S. Szilagyi, Helmar Bornemann-Cimenti. Nutrients. March 2023; doi: 10.3390/nu15061350. Quote: Chronic pain is a major source of morbidity for which there are limited effective treatments. Palmitoylethanolamide (PEA), a naturally occurring fatty acid amide, has demonstrated utility in the treatment of neuropathic and inflammatory pain. Emerging reports have supported a possible role for its use in the treatment of chronic pain, although this remains controversial. We undertook a systematic review and meta-analysis to examine the efficacy of PEA as an analgesic agent for chronic pain. A systematic literature search was performed, using the databases MEDLINE and Web of Science, to identify double-blind randomized controlled trials comparing PEA to placebo or active comparators in the treatment of chronic pain. All articles were independently screened by two reviewers. The primary outcome was pain intensity scores, for which a meta-analysis was undertaken using a random effects statistical model. Secondary outcomes including quality of life, functional status, and side effects are represented in a narrative synthesis. Our literature search identified 253 unique articles, of which 11 were ultimately included in the narrative synthesis and meta-analysis. Collectively, these articles described a combined sample size of 774 patients. PEA was found to reduce pain scores relative to comparators in a pooled estimate, with a standard mean difference of 1.68 (95% CI 1.05 to 2.31, p = 0.00001). Several studies reported additional benefits of PEA for quality of life and functional status, and no major side effects were attributed to PEA in any study. The results of this systematic review and meta-analysis suggest that PEA is an effective and well-tolerated treatment for chronic pain. Further study is warranted to determine the optimal dosing and administration parameters of PEA for analgesic effects in the context of chronic pain.
Combining co-ultramicronized palmitoylethanolamide and quercetin with physical therapy and acupuncture for chronic pain in a frail patient: a case report in a dog. Giorgia Della Rocca, Davide Gamba. Cureus: 16(9): a1323. September 2024. Quote: Introduction: Multimodal analgesia, i.e., combining different measures tailored to the individual patient and owner’s availability, is currently recognized as the ideal approach to successfully manage chronic pain, especially in frail veterinary patients. Palmitoylethanolamide (PEA) is a bioactive pro-homeostatic lipid mediator with anti-hyperalgesic properties, mainly due to the down-modulation of microglia and mast cells. Here we report on a frail dog with chronic pain managed with a multimodal non-pharmacologic protocol, including the dietary supplementation with a co-ultramicronized formulation of PEA and the antioxidant flavonoid quercetin (PEA-q, 5:1). Methods: An 8-year-old, 42 kg, intact male Labrador Retriever with IRIS stage 2 chronic kidney disease due to congenital polycystic kidneys was referred to our algology service because of long-lasting left thoracic limb lameness. At clinical visit, the dog showed pain at palpation of the neck, the right iliopsoas muscle, and the lumbosacral region. Two trigger points were bilaterally identified on the superficial gluteus muscle. At MRI, Hansen type-II disc protrusions were shown at C4-C5, C5-C6 and C6-C7. At x-ray, severe osteoarthritis of the lumbosacral and thoracolumbar spine was evident. Pain severity and pain interference were scored 6 and 5.3 out of 10, respectively, and quality of life was “poor” on the Canine Brief Pain Inventory (CBPI). Given the clinical frailty of the dog and the owner reluctance to drugs’ use (previously administered NSAIDs were poorly tolerated), pain was managed by associating the daily supplementation of PEA-q (14 mg/kg for 8 months) with physical therapy and acupuncture. Physical therapy (twice a week for the first 2 months and weekly for the following 6 months) consisted in TECAR therapy for 15 min (capacitive mode with bipolar handpiece power 50%) and laser therapy at10 Watt for 5 min and 15 s along the back, with a total energy of 2510 J. Five weekly acupuncture sessions were initially performed by placing needles in acupoints GV14, Bai Hui, LI4, BL (21, 23, 25 and 28 bilaterally) and ST36, as well as in correspondence of the areas of neck and gluteal muscle pain. For the following 7 months, 30-min electroacupuncture weekly sessions were applied bilaterally at acupoints BL 21, 23, 25 and 28 using a dense-disperse low frequency program (2 Hz, 210 μs for 4 s alternated with 100 Hz, 120 μs for 2 s). Results: Pain progressively decreased at monthly assessment on CBPI. Pain at palpation also gradually improved. At the end of treatment protocol, pain severity and interference on CBPI dropped to 3 and 3.5, respectively, and quality of life was assessed as “normal”. The treatment regimen was well tolerated. Conclusions: Long-term dietary supplementation with PEA-q, combined with a non-pharmacologic analgesic protocol, was shown to benefit a frail patient suffering from chronic pain. PEA-q may thus represent a further option for managing frail patients, because of its safety profile and the ability to target newly recognized mechanisms of chronic pain (i.e., neuroinflammation and oxidative stress).
Evaluation of the efficacy and Safety of Palmitoylethanolamide (PEA) combination in managing dermatitis in dogs. Harsh J Shah, Jacky K. Pariyani, Kalyani V. Shinde, Bhakti A. Dave, Priyal B. Dakhore. Int'l j. Vet. Sci. & Anim. Husb. May 2025; doi: 10.22271/veterinary.2025.v10.i5d.2261. Quote: Dermatitis is a common inflammatory skin condition in dogs, caused by allergies, infections, or autoimmune responses, leading to significant discomfort and reduced quality of life (QoL). This study evaluated the effectiveness and safety of Palmitoylethanolamide (PEA) combination-PEA 300mg + Daidzein 50mg + Genistein 4mg -in 18 [Labrador retriever] dogs suffering from dermatitis. Dogs received PEA Combination twice daily for 28 days and were assessed on day 0 (baseline), 07, 14, 21 and 28 (End of study). Key evaluations included Canine Atopic Dermatitis Extent and Severity Index-4 (CADESI-4) and owner-reported QoL. CADESI-4 reduced significantly from 52.27±5.51 to 3.83±2.89 (p<0.001). QoL score significantly improved from 13.33±2.11 to 0.61±0.97 (p<0.001), while family concern scores improved from 5.55±1.33 to 0.22±0.42. Two cases of vomiting were reported as adverse events. The study concluded that PEA combination significantly reduced dermatitis and improved QoL in dogs, demonstrating safety and tolerability with no major adverse event.
Pharmacokinetics of micronized and water dispersible palmitoylethanolamide in comparison with standard palmitoylethanolamide following single oral administration in male Sprague-Dawley rats. Mehkri, S; Dinesh, K. G.; Ashok, G.; Bopanna, Krathish. Indian J. Pharm. July 2025; doi: : 10.4103/ijp.ijp_964_24. Quote: Background: Palmitoylethanolamide (PEA) is an endogenous fatty acid amide signaling molecule that may indirectly modulate the endocannabinoid system. It has poor water solubility and, therefore, is not readily absorbed in the human body. Various formulation techniques have been developed to enhance the rate of dissolution of PEA and minimize the variability of drug absorption when administered orally. The present study compared the pharmacokinetics (PKs) of two different grades of micronized PEA, a water-dispersible PEA, and standard PEA in male Sprague–Dawley rats, following a single oral administration. Materials and Methods: The drug PKs of each form of PEA-micronized (PEA-10µm), micronized PEA 6 µm (PEA-6 µm), and water-dispersible PEA (PEA-WD), were assessed and compared against a standard nonmicronized PEA (PEA-nm) using male Sprague–Dawley rats after a single dose oral administration. PEA plasma concentration (ng/mL) across all groups was determined using the liquid chromatography with tandem mass spectrometry method. Results: PEA supplementation significantly increased the total area under curve (AUC) in all the groups compared to PEA-nm, with PEA-WD (P < 0.001) exhibiting an exceptionally large effect of > 16 times. ... The water-dispersible PEA proved effective in improving dissolution rate and bioavailability of poorly water-soluble PEA by over sixteenfold and was found to be much superior to the other forms of PEA reported in literature. ... In addition, the PEA supplementation raised the maximum concentration (Cmax) from the baseline in all the groups. When Cmax for each group was compared against PEA-nm at a probability level of 0.05, PEA-10 µm indicated no statistically significant difference (P = 0.060), whereas PEA-6 µm (P < 0.001) and PEA-WD (P < 0.001) displayed a statistically significant difference at the aforesaid probability level. Conclusion: PEA-WD demonstrated a higher total AUC and Cmax, followed by PEA-6 µm and PEA-10 µm compared to nonmicronized form of PEA. This indicates greater bioavailability of water-dispersible PEA, thus making it a promising candidate for enhancing clinical outcomes.
Pharmacokinetics of a natural hybrid-hydrogel formulation of palmitoylethanolamide (PEA): randomized, single-dose, double-blinded, 2-way crossover study. Maria Jose Louis, Ashil Joseph, Umesh Kannamangalam Vijayan, Abhilash Balakrishnan, Krishnakumar Illathu Madhavamenon. CyTA J. Food. July 2025; doi: 10.1080/19476337.2025.2516484. Quote: Palmitoylethanolamide (PEA) is an endogenous lipid having the ability to suppress inflammatory responses, restore homeostasis, and modulate pain sensitivity. However, its concentration in the body is often limited, especially on aging, owing to low production and rapid metabolism. PEA supplementation suffers from poor oral bioavailability and half-life. This study was aimed to characterize a natural self-emulsifying hybrid-hydrogel formulation of PEA (P-fen), developed using fenugreek galactomannan seed mucilage (FenuMat®), and further investigate its pharmacokinetics in healthy human volunteers, in comparison with micronized PEA (m-PEA). P-fen was an amorphous powder with a high swelling index and improved solubility (56-fold). It was observed as microspheres of 250 μm size with a porous and smooth surface upon scanning electron microscopy (SEM) analysis. Dynamic light scattering (DLS) analysis indicated ~523 nm particles, with a zeta potential of −60 mV indicating its aqueous stability. In vitro gastrointestinal studies revealed sustained release with better bioaccessibility (>80%). Pharmacokinetics following a single dose of P-fen revealed 5-fold enhancement in bioavailability.


CONNECT WITH US