Kidney Diseases in the Cavalier King Charles Spaniel
-
 Chronic
Kidney Disease
Chronic
Kidney Disease - Cardio-Renal Syndrome
- Protein-Losing Nephropathy
- Symptoms
- Diagnosis
- Stages of CKD
- Treatment
- Renal Dysplasia
- Symptoms
- Diagnosis
- Treatment
- RAAS Activation
- Acute Kidney Injury
- Fanconi Syndrome
- Breeders' Responsibilities
- What You Can Do
- Research News
- Related Links
- Veterinary Resources
A dog's kidneys play a major role in the blood circulatory system, filtering the blood and removing waste products*, and regulating blood pressure. The kidneys receive about 25% of the cardiac output. Kidneys are essential to the urinary system. They produce highly concentrated urine which efficiently excretes a large quantity of toxins in a relatively small quantity of water. Kidney failure -- or renal failure -- occurs when the kidney cannot filter the toxins and other wastes in concentrated urine.
*Waste products include urea nitrogen, creatinine, nitrogen waste products, phosphorus, symmetric dimethylarginine (SDMA), hemoglobin breakdown products, and hormone metabolites.
The functions of the kidneys are to (a) maintain the body's pH, (b) reabsorb nutrients, (c) regulate blood pressure, (d) excrete wastes, (e) remove excess fluid from the body, and (f) secrete hormones for production of red blood cells and acid regulation. The structural and functional units of the dog's kidneys are roughly 200,000 nephrons, which are tube-like structures which filter and purify the blood by removing solid and liquid waste products before they are excreted as urine.
Here we discuss chronic kidney disease (more specifically, cardio-renal disorders), which is more prevalent in the cavalier King Charles spaniel than in most other breeds. Here, we also discuss renal dysplasia, a less common disorder in cavaliers, which is congenital, meaning present at or soon after birth. In a separate article, we discuss another kidney disorder prevalent in the CKCS, called xanthinuria, which are crystals or sediment in the dogs' urinary tract.
RETURN TO TOP
Chronic Kidney Disease
Chronic kidney disease* (CKD) is defined as a progressive loss in kidney function, by the loss of nephrons, over a period of three or more months. Nephrons are not reproducible, so as they are damaged or lost, the kidneys' functions decline. Cavalier King Charles spaniels and Cocker spaniels reportedly are at increased risk to develop CKD, according to a July 2013 UK study.
* CKD is also referred to as chronic renal disease. "Renal" refers to kidney functions.
RETURN TO TOP
• Cardio-Renal Syndrome
In addition, CKD may develop as a consequence of either deteriorating cardiac function or the administration of diuretics, such as furosemide and torsemide, which can severely affect the kidney by reduced blood flow to the kidneys and altered renal tubular function and by activating the renin-angiotensin aldosterone system (RAAS), since reduction in the total circulating blood volume results in activation of RAAS. Further, deteriorating renal function can lead to cardiac dysfunction. This connection of cardiac dysfunction and renal dysfunction has been referred to as "cardio-renal syndrome" (CRS). CRS is defined as "disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other."
Cardio-renal syndrome (CRS) has been categorized into five types, depending upon the initial cause and its severity, in this November 2008 article, as shown in this chart:
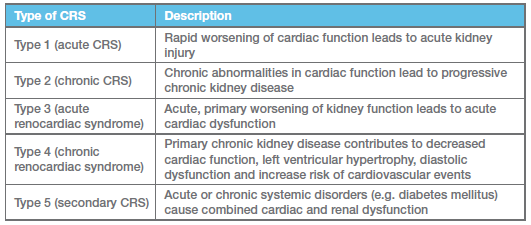
Renal dysfunction develops over time in cardiac patients, usually increasing incrementally as the cardiac disease progresses, then increasing more swiftly when cardiac medical therapy, particularly after diuretics and some vasodilators commence. As the CRS progresses from Type 1 to Type 5, additional renal disorders develop, such as the onset of glomerulonephritis in Type 3, a protein-losing nephropahty (PLN).
Furosemide and other diuretics can severely affect the kidneys by activating the renin-angiotensin aldosterone system (RAAS)*, since reduction in the total circulating blood volume results in activation of RAAS.
* When the RAAS is activated, it causes the kidneys to over-work by retaining more water and sodium and excreting more potassium. As a result of this process, the overall volume of blood increases, meaning that more blood is pumped through narrowed arteries, which also increases the blood pressure.
In some cases, inadequate therapy of venous congestion may contribute to renal dysfunction, resulting in congestive kidney failure. See this October 2011 presentation for details.
In a September 2015 report, a team of 9 veterinary cardiologists and 7 veterinary nephrologists from Europe and North America have issued a "Consensus Statement" to increase the awareness of and codify the definition, classification, diagnosis, and management strategies for veterinary patients with CRS, with an emphasis on the pathological interplay between the two organ systems. They acknowledge "a growing understanding of the complexity of interplay between renal and cardiovascular systems in both health and disease."
 They
have observed: "The concept of CRS, which involves a bidirectional
pathway of injury wherein disease of either organ directly or indirectly
contributes to injury of the other." And they have included a chart
showing postulated mechanisms underlying the relationship between heart
failure (HF) and renal dysfunction. (Click on the thumbnail chart at
right.)
They
have observed: "The concept of CRS, which involves a bidirectional
pathway of injury wherein disease of either organ directly or indirectly
contributes to injury of the other." And they have included a chart
showing postulated mechanisms underlying the relationship between heart
failure (HF) and renal dysfunction. (Click on the thumbnail chart at
right.)
Among their consensus statements, are:
"Statement 8: Thoracic radiography is recommended to assess the presence or absence of congestive heart failure, and echocardiography is recommended to assess cardiac morphology, lesions, and to estimate relevant haemodynamic parameters.
"Statement 9: Renal imaging is recommended to improve diagnosis, prognosis and guide potential therapies in CvRD. Conventional abdominal radiographs and ultrasound are recommended to help detect morphological abnormalities and determine underlying aetiology.
"Statement 10: As the kidney and heart are two organs at risk for damage due to systemic hypertension, and as kidney disease is often associated with systemic arterial hypertension, systemic arterial blood pressure should be systematically monitored in both kidney and cardiovascular diseases. * * *
"Statement 13: ... particular attention should be directed towards the following when managing any form of [CRS]: 1) identification and treatment of elevated blood pressure as per IRIS recommendations; 2) stepwise titration of dosages of diuretics, ACEI, inotropes and/or fluids with frequent monitoring of renal function, body weight, hydration, electrolyte status, and systemic blood pressure (i.e. performed and rechecked within 3-5 days following initiation or dose adjustment of these drugs); 3) proper nutrition, with respect to reduced dietary sodium and phosphate and appropriate protein and caloric intake."
RETURN TO TOP
• Protein-Losing Nephropathy (PLN)
Protein-losing nephropathies (PLN) include glomerulonephritis (GN), glomerulopathy, and amyloidosis. The glomerulus is a cluster of blood capillaries at the end of each kidney tubule, which when functioning normally, freely filters through small molecules of urea, creatinine, sodium, etc. but blocks passage of larger molecules, such as albumin.
Glomerulonephritis (glomerular nephritis -- GN) is inflammation of the glomeruli. When the glomeruli filter out immune complexes of antibodies and antigens from the blood, and they become trapped in the glomeruli, the immune defenses are activated, causing damage to the glomeruli. See this November 2013 Consensus Statement on glomerular disease and this April 2016 article.
RETURN TO TOP
• Symptoms
In CKD's earliest stages, the dog may urinate more often than
usual, or in larger quantities, due to the failure of
 the kidneys to concentrate
the urine. This results in dehydration and thirst, so the dog tends to
drink more water than usual. In the
July 2013 UK
study of over 100,000 dogs, the most significantly associated clinical signs
included:
the kidneys to concentrate
the urine. This results in dehydration and thirst, so the dog tends to
drink more water than usual. In the
July 2013 UK
study of over 100,000 dogs, the most significantly associated clinical signs
included:
• halitosis (bad breath)
• anemia
• weight loss
• decreased muscle mass (cachexia)
• excessive urination
• excessive thirst
• urinary incontinence
• vomiting
As the kidneys' function decreases, any of several symptoms may appear. Blood pressure may increase, due to an overload of fluids and the production of vasoactive hormones created by the kidneys' renin-angiotensin aldosterone system (RAAS). Urea may accumulate, leading to azotemia (an increased concentration of nonprotein nitrogenous compounds, usually urea and creatinine, in blood), followed by uremia (retention of ammonia, nitrogen, acids, and other chemical wastes in the blood and tissues), with additonal symptoms:
• apathy
• depression
• loss of appetite and weight
• a dry haircoat
• brownish discoloration of the tongue
• an ammonia odor to the breath
• drinking less than normal.
As the kidneys' efficiency declines, fluid volume will overload, with symptoms of various levels of pulmonary edema and pericarditis (an inflammation of the pericardium, the fibrous sac surrounding the heart). Urea could be excreted through the skin. Potassium levels may increase in the blood (hyperkalemia) with symptoms varying from fatigue to cardiac arrhythmias. Ulcers may appear in the mouth. Vomiting, diarrhea, and gastrointestinal bleeding may occur. At the end stage of kidney failure, the dog has seizures and will fall into a coma.
Kidney dysfunction is tied closely to heart failure, and for the cavalier King Charles spaniel in particular, mitral valve disease (MVD) and CKD go hand-in-hand. In the July 2013 UK study, cardiac disorders were among the most common co-diseases with CKD.
RETURN TO TOP
• Diagnosis
 CKD may be classified in five stages, with Stage 1 being the
mildest, with few symptoms, and Stage 4 being the most severe and often called
chronic kidney failure (CKF) or chronic renal failure (CRF) or end stage renal
disease (ESRD). See more information about these stages
below.
CKD may be classified in five stages, with Stage 1 being the
mildest, with few symptoms, and Stage 4 being the most severe and often called
chronic kidney failure (CKF) or chronic renal failure (CRF) or end stage renal
disease (ESRD). See more information about these stages
below.
The September 2015 Consensus Statement issued by the international team of veterinary cardiologists and nephrologists, includes these diagnosis recommendations:
"Statement 8: Thoracic radiography is recommended to assess the presence or absence of congestive heart failure, and echocardiography is recommended to assess cardiac morphology, lesions, and to estimate relevant haemodynamic parameters.
"Statement 9: Renal imaging is recommended to improve diagnosis, prognosis and guide potential therapies in CvRD. Conventional abdominal radiographs and ultrasound are recommended to help detect morphological abnormalities and determine underlying aetiology.
"Statement 10: As the kidney and heart are two organs at risk for damage due to systemic hypertension, and as kidney disease is often associated with systemic arterial hypertension, systemic arterial blood pressure should be systematically monitored in both kidney and cardiovascular diseases."
Urinalysis
Chronic kidney disease is detected by urinalysis (e.g., dipstick, sediment) including urine specific gravity (USG) to determine how well the dog is concentrating urine and for protein levels, and by a Complete Blood Count (CBC) and Blood Chemistry Panel, showing any increase in blood urea nitrogen (BUN).
Uremia is defined as the buildup of waste products in the blood. Symptoms associated with unremia may include nausea, vomiting, fatigue, anorexia, weight loss, muscle cramps, pruritus, and/or changes in mental behaviors.
RETURN TO TOP
Creatinine
Serum creatinine (sCr) is a biomarker test for kidney disease and injury. Higher levels of creatinine indicate a lower flow rate of fluids through the kidneys (glomerular filtration rate -- GFR) and as a result a decreased capability of the kidneys to excrete waste products. Creatinine levels may be normal in the early stages of CKD, and the condition is discovered if urinalysis shows that the kidneys are allowing the flow of proteins or red blood cells into the urine. A downside of this testing is that creatinine does not increase until 75% of kidney function is lost.
In a July 2018 article,, Spanish researchers reported finding that salivary urea and creatinine concentrations were significantly higher in 19 dogs affected with chronic kidney disease (CKD) compared with 17 healthy dogs. They found that urea and creatinine concentrations can be measured in canine saliva with commercially available spectrophotometric assays. Both assays showed higher values in saliva of dogs with CKD compared with healthy dogs and their values were highly correlated with those in serum.
RETURN TO TOP
Blood urea nitrogen (BUN)
Blood urea nitrogen (BUN) is a blood test of kidney function. The "normal" range for BUN in dogs is a value from 10 to 30. As kidney disease begins and progresses, the BUN level usually increases. An elevated BUN level alone may not indicate any kidney disorder. For example, dogs fed raw or homemade recipes of food typically have higher BUN levels than are considered normal, such as in the range of 40. This would be due mainly to the freshness of the meats the dogs consume in such diets. Mild dehydration also may cause an elevated BUN level.
A non-kidney related disorder casusing high BUN levels is hemorrhaging due to ulceration in the gastro-intestinal tract-- hemorrhagic gastroenteritis.
Azotemia is defined as an elevation of blood urea nitrogen (BUN) and serum creatinine levels.
RETURN TO TOP
Symmetric Dimethylarginine (SDMA)
Symmetric dimethylarginine (SDMA) is another biomarker test for kidney disease and injury. In January 2015, Idexx Laboratories announced a biomarker test for CKD, called symmetric dimethylarginine (SDMA), which reportedly enables diagnosis of CKD several months or even years earlier than creatinine tests. SDMA is a methylated form of the amino acid arginine, which is released into the circulation during protein degradation and is excreted almost exclusively by the kidneys. SDMA is a biomarker used to assess glomerular filtration rate in the diagnosis, classification, and monitoring of CKD. SDMA reportedly increases earlier than creatinine in CKD; it begins increasing as early as 25% loss of kidney function, and on average with 40% loss of kidney function versus creatinine, which does not increase until 75% of kidney function is lost. See also this March 2016 article and this November 2020 article discussing symmetric dimethylarginine.
In a September 2016 study by a team of Korean cardiology researchers, they examined serum concentration levels of two kidney function biomarkers -- Cystatin-C or cystatin 3 (Cys-C) and symmetric dimethylarginine (SDMA). They confirmed the findings of previous studies involving SDMA, noting that SDMA does not appear to be influenced by age, body weight or gender. Regarding Cys-C, they disagreed with prior studies findings that the Cys-C concentration was influenced by age and body weight. They stated, "However, our study strongly suggested that the Cys-C was not influenced by age or body weight." Nevertheless, they concluded that, "Although the Cys-C is a promising renal marker for canine kidney disease, test standardization and reference range have yet been clearly established in dogs."
In an August 2018 article, Italian researchers reported that:
"In conclusion, our study showed that SDMA is free from correlation with breed, age, sex, weight, presence/absence of MMVD, presence of CHF symptoms and pharmacological therapy as well. SDMA can be actually considered a reliable parameter for evaluation of renal function in dogs affected by MMVD, especially in those patients with a non-advanced stage of disease (ACVIM class B2), for which an early diagnosis of the onset of kidney failure is fundamental in order to plan a diuretic therapy. SDMA repeated measurements over time, as recommended by IRIS guidelines, are necessary (IRIS, 2016), because one determination does not allow us to exclude definitely a later onset of the renal impairment and then to be considered diagnostic in order to highlight a possible onset of CRS [cardiorenal syndrome]."
In a September 2020 article, a team of Italian and Swiss researchers attempted to determine if SDMA increased as mitral valve disease (MVD) progressed from Stage B1 to Stages C and D in 78 MVD-affected dogs (including three cavaliers). They claim to have categorized the MVD-affected dogs according to the 2019 ACVIM definitions of Stages B1 and B2. However, they misstate those definitions, and so it is not possible to determine whether any of the dogs placed in Stage B2 met the 2019 ACVIM version or not. The 2019 ACVIM Stage B2 requires that both the left atrium and left ventricle echocardiographic measurements meet or exceed a specific minimum cut point. In this study, dogs were placed in Stage B2 if either the left atrium or left ventricle met its minimum cut point. The researchers conclude:
"In conclusion, the results of this study failed to demonstrate that renal function, evaluated by measuring serum SDMA concentration, is significantly impaired in dogs with MMVD. Although some dogs in the ACVIM Stages] C+D group of MMVD had an increased concentration of the variables used to identify renal dysfunction, this was most likely due to pre-renal azotemia instead of representing a feature of the CRS [cardiorenal syndrome] described in humans."
RETURN TO TOP
Cystatin B
Cystatin B (Cys-B) is another biomarker for kidney disease and injury. It is a protein present in the cells of the renal tubular epithelium. When the renal tubules are affected, cystatin B is released into the dog's urine. IDEXX's Cystatin B Test, which is urine-based, can detect unjury to the renal tubules before clinical signs appear.
RETURN TO TOP
Cystatin C
Cystatin C (Cys-C) is another biomarker for early detection of renal dysfunction. It is a low-molecular-weight protein that is produced by cells throughout the body and is filtered out of the blood by the glomeruli in the kidneys and then broken down and reabsorbed by the renal tubules. Studies have shown that Cys-C is a more sensitive and specific marker for early renal dysfunction than other markers, such as serum creatinine. Cys-C also may be a biomarker for identifying dogs at risk of mitral valve disease (MVD) and for monitoring disease progression. See this November 2023 article.
RETURN TO TOP
Imaging
Tomography (a form of medical imaging) and renal biopsies may be performed to determine the cause for the kidneys' malfunction. Alternatively, an abdominal ultrasound may be performed. Intrarenal Doppler ultrasonography (IRD) is used to evaluate blood flow through the veins in the kidneys (hemodynamics), a measure of renal congestion. Contrast-enhanced ultrasonography (CEUS) also evaluates intrarenal hemodynamics, using microbubble-based contrast agents.
In a November 2024 article, CEUS was used to examine renal perfusion in 23 dogs diagnosed with mitral valve disease, including 4 cavaliers (17.4%), compared to a control group of dogs with healthy hearts. The rise times of the renal cortex and medulla were measured from a time-intensity curve. The rise time of the cortex was longer in dogs with stage B2 MVD than in control dogs, while that of the medulla was shortened in the right ventricular dysfunction group in stage B2. No changes were observed in IRD indices (the resistance index and venous impedance index). The researchers concluded that CEUS detected changes in renal perfusion in dogs with preclinical (Stages B1 and B2) MVD even when IRD indices remained unchanged, suggesting the utility of CEUS in evaluations of renal perfusion in MVD dogs.
RETURN TO TOP
• Stages of CKD
The International Renal Interest Society (IRIS) has defined four "Stages" of CKD in dogs, ranging from the mildest, Stage 1, to the most severe, Stage 4. The stages are determimed by the blood testing levels of creatinine and Symmetric dimethylarginine (SDMA). According to the IRIS, the stage of CKD should determine the type of treatment most appropriate for the patient.
Stage 1: If the blood creatinine level is <125 μmol/l or <1.4 mg/dl and the SDMA level is <18 μg/d, the creatinine level is normal and the SDMA level may be mildly increased. If some abnormal renal symptom exists (such as inadequate urinary concentrating ability, abnormal renal palpation or renal imaging findings, proteinuria of renal origin, abnormal renal biopsy results) and/or if recent SDMA levels have been >14 μg/dl persistently, then early CKD may be diagnosed.
Stage 2: Increased creatinine levels at 125-250 μmol/l or 1.4-2.8 mg/dl and SDMA at 18-35 μg/d, with mild renal azotemia. Any observable symptoms (clinical signs) are usually either mild or absent.
Stage 3: Increased creatinine levels at 250-440 μmol/l or 2.9-5.0 mg/dl and SDMA at 36-54 μg/d, with mild azotemia. Clinical signs of CKD may be present in any of varying degrees. If no signs are observed, the dog would be classified as in "early Stage 3". Extreme signs would mean "late Stage 3".
Stage 4: Increased creatinine levels at above 440 μmol/l or above 5.0 mg/dl and SDMA above 54 μg/d. Typical symptoms of CKD would be present, and the dog would suffer from uremia.
RETURN TO TOP
• Treatment
There is no cure for chronic kidney disease (CKD). The veterinarian's goal is to control the disease as well as possible for the rest of the dog's life. Restricting the CKD dog's salt intake, to help prevent edema, ascites, and hypertension, likely will be prescribed. Periodic blood tests will be recommended, to detect changes in kidney function and the progression of the disease.
The September 2015 Consensus Statement issued by the international team of veterinary cardiologists and nephrologists, includes these treatment recommendations:
"Statement 13: ... particular attention should be directed towards the following when managing any form of [CRS]: 1) identification and treatment of elevated blood pressure as per IRIS recommendations; 2) stepwise titration of dosages of diuretics, ACEI, inotropes and/or fluids with frequent monitoring of renal function, body weight, hydration, electrolyte status, and systemic blood pressure (i.e. performed and rechecked within 3-5 days following initiation or dose adjustment of these drugs); 3) proper nutrition, with respect to reduced dietary sodium and phosphate and appropriate protein and caloric intake."
In 2023, the International Renal Interest Society (IRIS) issued its "Treatment Recommendations for CKD in Dogs", which goes into great detail on how to treat in each IRIS stage of CKD.
Nutrition
Dietary therapy is a primary method of managing kidney diseases. Specially designed renal diets should take into account several necessary forms of management of CKD:
• Modified amounts of high-quality proteins
• Increased calories and fiber
• Vitamins and supplements, e.g., Omega-3 fatty acids and antioxidants
• Reduced amounts of phosphorus and sodium
• Increased moisture content
Medicus Kidney Support Diet: This is a dog food designed to support the kidneys of CKD-affected dogs. It requires a veterinarian's prescription.
Often, affected dogs may find renal diets unappetizing, and therefore the dogs refuse to eat them. One of the main factors in fashioning a renal diet is consumption of adequate calories. Insufficient caloric intake may lead to malnutrition, particularly of proteins, resulting in a decrease of muscle mass and anemia. See Appetite Stimulants, below. An extreme solution to unacceptance is a feeding tube.
Some nutrients and foods have been found to cause the progression of CKD and therefore must be avoided, while others may delay that progression and are to be added to the daily diets. Also, some nutrients and foods may serve to relieve symptoms of CKD and improve the affected dog's quality of life.
Moisture: Sufficient quantities of water must always be available. The dog must be able to take in enough water to compensate for large urine output. The affected dog's food should include 70% to 85% moisture. Therefore, any dry foods (kibble) should be avoided, for this reason and others discussed below. The injection of fluids under the skin, known as subcutaneous ( sub-Q or SQ) fluids, may be necessary.
Protein: Restriction of protein in the dog's diet, to delay the progression of CKD, is the subject of great controversy, with research investigators reporting conflicting results of protein restrictions. The opinion among some veterinary authors of published articles on CKD-affected dogs is that reducing dietary protein likely will cause other chronic health problems, especially lean muscle mass loss, which otherwise could be prevented. Therefore, to avoid excessive reduction of proteins in the renal diets, as well as to avoid high phosphorus levels (see Phosphorus, below), alternatives to meats may be included, such as eggs, which are both high in protein and very low in phosphorus, along with certain protein-rich grains such as barley, and legumes.
Alternatively, energy-dense diets may be substituted for protein, but this is not recommended over a long time period.
Phosphorus: Dogs with CKD have been found to not be able to process phosphorus properly, so the excess phosphorus builds up in their blood. Two ways to remove the excess phosphorus is (1) restrict the amount of phosphorus in the affected dog's diet, and (2) add phosphorus binders, such as chitosan and calcium citrate to the dog's meals. Some studies have found that restricting the phosphorus content in the dogs' diets (no more than 0.2% to 0.4% per meal) has led to less renal damage and extended life-times of CKD-affected dogs. See this 1999 report and this 2008 report. Eggs are both high in protein and very low in phosphorus and therefore enable recipes to maintain protein levels while reducing phosphorus levels. Medications to lower phosphorus levels also may be prescribed, including enteric phosphate binders such as calcium-rich Renal P by Candioli Pharma.
Sodium: As noted above, sodium intake should be restricted only moderately. In the September 2015 Consensus Statement, the team of veterinary cardiologists and nephrologists recommend only "moderately" limiting sodium intake, reduced phosphorus intake, and adequate protein to support the needs of MVD-affected dogs. Specifically, they stated:
"Ensuring proper nutrition is an important component of managing CvRD [cardiovascular-renal disorders]. Moderately sodium-restricted diets are appropriate for both kidney and cardiovascular diseases and reduced phosphorus diets are important in kidney disease. As previously mentioned, dogs with chronic heart disease may lose muscle mass and body condition, thus confirming that adequate protein and caloric intake is an important goal. In animals with CvRD, this need is counterbalanced by the detrimental effect of high protein intake on azotaemia; therefore, careful dietary planning with the support of a veterinary nutritionist or internist might be helpful. Dietary supplements, such as omega-3 fatty acids, are occasionally used in animals with CvRD, both as antioxidants and appetite stimulants, but their safety and efficacy have not been rigorously demonstrated."
Excessive reduction of sodium in some commercial "prescription" diets, such as Hill's Prescription Diet h/d dry food, can actually activate the kidneys' RAAS and cause added damage on top of the affected dog's CKD. See this July 2022 article and this Novermber 2024 article.
Dry dog foods -- kibble: Dry foods in general have the potential of causing or contributing to renal failure and are not appropriate for treating kidney disease. Reasons for not feeding dogs dry foods if CKD is at hand or a concern are:
• There is no moisture in dry foods, and CKD-affected dogs desperately need water in their diets.
• Dry foods rely heavily upon grains and other carbohydrates for both protein sources and to hold the kibble pieces together.
• The meats (such as they are) in dry foods are heavily processed, making the kidneys work harder.
• Mold build-up in dry foods is a common problem, producing fungal toxins such as Ochratoxin.
• High sodium levels in dry foods.CKD dogs need some sodium but not as much as is in most dry foods.
• Dry foods tend to be loaded with synthetic supplements and other chemicals requring the kidneys to work overtime to filter them.
On the other hand, some dry foods are so low in sodium content that they activate the RAAS, which also can cause renal failure. See this July 2022 article and this Novermber 2024 article.
 Appetite
Stimulants:
Treating veterinarians may
prescribe an appetite stimulant, such as mirtazapine (Remeron) or
meclizine
(Antivert, Bonine, Dramamine II, Driminate II), or
capromorelin (Entyce) or
Azodyl.
Appetite
Stimulants:
Treating veterinarians may
prescribe an appetite stimulant, such as mirtazapine (Remeron) or
meclizine
(Antivert, Bonine, Dramamine II, Driminate II), or
capromorelin (Entyce) or
Azodyl.
In a November 2016 article by a team of French specialists, they reported on the use of a palatable high-energy complementary feed composed of high-energy ingredients (glucose syrup, soybean oil and cod liver oil), hydrolyzed animal proteins, vitamins and oligo-elements, called Nutri-Plus Gel, in five case studies.
The USA's Food & Drug Administration (FDA) has approved the canine appetite stimulant Entyce (capromorelin). Capromorelin is a ghrelin receptor agonist which mimics the activity of ghrelin, a hormone which reportedly causes a sense of hunger.
RETURN TO TOP
Supplements
Fish oil (for Omega-3 fatty acids in the form of EPA and DHA) as a dietary supplement has been found to be beneficial to CKD-affected dogs.
A general kidney support supplement often recommended by holistic
veterinarians is
Canine Renal Support by Standard Process.
Renal K+ (potassium gluconate), by Vetoquinol USA, is
an oral powder
 potassium supplement to support the kidneys.
potassium supplement to support the kidneys.
Chitosan ( is a sugar that serves as a binder with phosphorus, limiting its absorption and removing it from the dog's body. It is derived from chitin, which is found in cell walls of crabs, lobster, and shrimp, among other sources. Another phosphate binder containing chitosan is Nutramax' Naraquin, which some veterinarians are prescribing for dealing with high phosphorus levels.
Some veterinarians also are recommending Azodyl, a supplement which contains strains of three naturally occurring bacteria (S. thermophilus, L. acidophilus, and B. longum) and a prebiotic which reportedly supports kidney detoxifiation. It may be used once azotemia (an increased concentration of nonprotein nitrogenous compounds, usually urea and creatinine, in blood) is detected. It has been found to increase the affected dog's appetite and reduce BUN levels, starting at about four weeks after first administering it.
Zhen wu tang is a is a traditional Chinese proprietary blend of herbs (porta, radix paeoniae alba, rhizoma zingiberis recens, rhizoma atractylodis macrocephalae, and radix aconiti lasteralis praeparata) which is designed to treat kidney disorders which may cause renal failure.
Some types of kidney failure are acute, and are mild enough that if the dog is well supported medically, there will be a complete recovery. More commonly, dogs will have at least some renal function deficit and need a change in care for the rest of their lives.
RETURN TO TOP
Dialysis
Peritoneal dialysis, which supplements the filtering tasks of the kidneys, may be advised. In peritoneal dialysis, fluids are fed into the abdomen using a catheter, to allow them to perform the filtering process, after which, the fluids are removed through the catheter, taking filtered toxins out with them. Hemodialysis is a technique in which the dog's blood is circulated through a filtering machine.
Continuous renal replacement therapy (CRRT) is a slow and gradual blood purification procedure, in which the dog's blood is filtered to remove toxins and then returned to the dog's body through a catheter. The process involves blood purification filtration, adding a fluid (dialysate) which removes toxins from the filter, and adding a sterile replacement fluid which flushes toxins from the body and replaces electrolytes and other blood elements.
RETURN TO TOP
Surgery
Kidney transplants are a rare option.
RETURN TO TOP
Renal Dysplasia
Renal dysplasia is a defect in the development of one or both of the dog's kidneys while still a fetus in the womb. Cavalier King Charles spaniels are among the breeds in which this congenital disorder has been reported to be more prevalent than the average dog.* The possible causes may be due to an infection in the womb, such as canine herpesvirus, unfavorable environmental conditions called teratogen agents, as well as familial (blood line) and inheritance.
The defective kidney(s) result in the inability to remove toxins in the blood.
* See these veterinary articles: May 1989; March 2001; 2003; July 2005; 2015; May 2022.
RETURN TO TOP
• Symptoms
Signs of renal dysplasia may become apparent soon after birth, but usually between 6 months and 2 years of age. Symptoms generally are the same as those of chronic kidney disease (CKD), but may also include failure to thrive at birth. Others are:
• excessive urination (polyuria)
• urinary incontenence
• excessive thirst (polydipsia)
• vomiting
• weight loss
• anorexia
• lethargy
• anemia
• bad breath (halitosis)
Severe cases of renal dysplasia, due to the buildup of toxins in the blood, may result in:
• diarrhea
• vomiting of dried blood
• weakness and collapse
• tremors in muscles
• neurological signs
RETURN TO TOP
• Diagnosis
Diagnosis for renal dysplasia would be the same as for chronic kidney disease (CKD). X-rays of the abdomen may show small or irregular shapes of the kidneys and skeletal deformities resulting from the dysplasia.
RETURN TO TOP
• Treatment
There is no known cure for renal dysplasia. Even with treatment, the prognosis for recovery is not good, due to the progressive deterioration of the condition. Otherwise, the treatment options are the same as those for chronic kidney disease (CKD).
RETURN TO TOP
RAAS Activation
The renin-angiotensin-aldosterone system (RAAS) is described as a "cascade" or chain-reaction which narrows the blood vessels and causes the kidneys to retain water and sodium, thereby increasing the amount of fluid in the dog's body and raising its blood pressure.
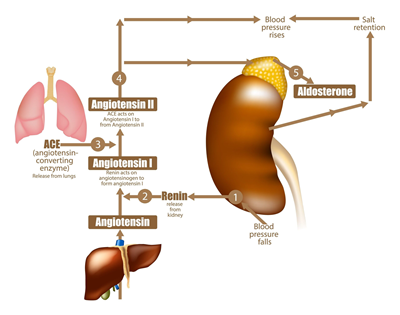 RAAS activation begins with renin, a combination of
amino acid residues which form a peptide, which helps control blood
pressure levels and also levels of sodium and potassium in the body.
Renin is secreted by the kidneys into the blood stream when receptors in
the dog's arteries detect low blood pressure or when the kidneys detect
low levels of sodium (salt) in the dog's blood.
RAAS activation begins with renin, a combination of
amino acid residues which form a peptide, which helps control blood
pressure levels and also levels of sodium and potassium in the body.
Renin is secreted by the kidneys into the blood stream when receptors in
the dog's arteries detect low blood pressure or when the kidneys detect
low levels of sodium (salt) in the dog's blood.
When renin is released into the blood stream, it triggers that cascade of events. Initially, it causes the formation of angiotensin I (Ang I). Angiotensin is a peptide homone that serves to narrow (constrict -- vasoconstrict) the blood vessels when appropriate to increase blood pressure. There are four different forms of angiotensin, angiotensin I, II, III, and IV. The next step in the cascade is for angiotensin converting enzyme (ACE) to cleave to the angiotensin I (Ang I) and convert it into angiotensin II (Ang II), which is the main and active form of the hormone and which results in higher blood pressure and increased sodium levels. Ang II also can be produced "locally" in the heart, blood vessels, adrenal glands, kidneys, and other tissues.
Ang II stimulates the release of aldosterone, which is a mineralocorticoid steroid hormone produced by the adrenal gland. It serves to conserve sodium and regulate potassium levels and blood pressure. Ang II also acts in the brain by binding to the hypothalamus to increase the dog's thirst and appetite for salt.
RETURN TO TOP
• Causes of RAAS activation
While activation of the RAAS can be a normal and appropriate process to compensate for abnormally low blood pressure or low sodium levels, chronic and unwanted RAAS activation can occur in response to dietary salt restriction, diuretic treatment, dehydration, and sometimes even strenuous exercise.
In a July 2022 article, nine healthy dogs fed a low-sodium diet of Hill's Prescription Diet h/d dry food for five days. Their levels of sodium reached such low levels that it caused the dogs' RAAS to steadily activate.
RETURN TO TOP
• Disorders caused or aggravated by RAAS
Activation of the RAAS has been identified as either causing or aggravating chronic kidney disease (CKD), excessively high blood pressure, proteinuria (excess of proteins in the blood).
Most significantly for dogs diagnosed with mitral valve disease (MVD), activated RAAS counteracts the effects of MVD medications, particularly diuretics given to MVD-affected dogs in heart failure (Stages C and D). Indeed, the reason that certain MVD medications are prescribed is to defend against the effects of activated RAAS. They include ACE-inhibitors and angiotensin receptor blockers (ARBs).
RETURN TO TOP
Acute Kidney Injury
Acute kidney injury (AKI) describes a range of renal injuries from mild to severe acute renal failure. The International Renal Interest Society (IRIS) has defined and graded AKI in a handout linked here. The IRIS AKI Grading scale (I-V) for dogs is based on fasting blood creatinine determination and clinical parameters, such as urinary flow rate.
Acute kidney injury (AKI) may be a common side effect following cardiac-bypass surgeries in MVD-affected dogs. In a September 2022 article, a team of investigators at the Royal Veterinary College reported finding acute kidney injury (AKI) in 3 of 19 dogs (15.8%) which were in recovery following cardiac surgery under cardiopulmonary bypass (CPB). Of the 19 dogs, 7 were cavaliers (37%), although the report does not indicate which dogs developed AKI. Also, two of the dogs died during the post-operative period prior to discharge from the hospital. They report finding that specific kidney biomarkers -- inosine and urinary cystatin B (uCysB) -- changed significantly following the surgeries, potentially indicating tubular injury of the kidneys. They determined that MVD-affected dogs undergoing surgeries under CPB are "at increased risk of AKI", and that biomarkers may promptly identify AKI in order to implement preventative and theraputic treatments.
The IDEXX Cystatin B Test is a urine test that can detect active renal tubular injury without any apparent clinical signs.
Extracorporeal therapy, for dogs with kidney injuries, may be performed in some clinics. It is a form of dialysis called renal replacement therapy. It removes toxins using various filters which are sized to remove different sizes and types of molecules.
RETURN TO TOP
Fanconi Syndrome
Fanconi syndrome is the short-hand name of a kidney disorder formally known as acquired canine proximal renal tubulopathy. It is associated with the ingestion of toxic substances, such as copper, lead, ethylene glycol, and some medications, such as gentamycin and amoxicillin. Recently, dogs which have eaten certain types of jerky treats, especially treats those based on chicken and originating from China, have developed Fanconi syndrome.
Urinalysis is an essential diagnostic tool in detecting Fanconi Syndrome.
In a November 2022 article, four cavaliers (13.3%) among a group of 30 dogs of various breeds in Denmark, were diagnosed with Fanconi syndrome after having been fed such jerky treats. Symptoms of the affected dogs included (a) excessive or abnormal thirst (polydipsia), (b) excessive urination (polyuria), (c) lethargy, (d) weight loss, (e) decreased appetite (hyporexia), (f) vomiting, and (g) diarrhea. All 30 of the dogs had abnormal levels of sugar in their urine. Treatment of the disorder varied from just discontinuation of feeding the jerky to a variety of symptom medications or dietary changes or supplements.
RETURN TO TOP
Breeders' Responsibilities
Since renal dysplasia in cavaliers appears to be an inherited condition due to its reported prevalence in the breed, the dog's breeder should be notified of the diagnosis, and the birth sire and dam and littermates should be examined to determine heritability.
RETURN TO TOP
What You Can Do
Since chronic kidney disease and renal dysplasia are more prevalent in cavaliers
than in other breeds, the dog's
 urine and blood should be routinely tested as a part of the dog's
regular veterinary examinations, from puppyhood. Periodic urine and
blood testing
can serve as an early warning system for future kidney issues.
urine and blood should be routinely tested as a part of the dog's
regular veterinary examinations, from puppyhood. Periodic urine and
blood testing
can serve as an early warning system for future kidney issues.
By all means, avoid feeding dry foods (kibble). They either have too much or too little sodium. Either way, they can activate the RAAS and start down the road to destroying the kidneys.
RETURN TO TOP
Research News
December 2023:
Cavaliers ranked first among 50 dogs with combined renal proteinuria and
blood clots in arteries.
 In
a
December 2023 article, Royal Veterinary College (RVC) researchers
Luca Fortuna (right) and Harriet M. Syme studied the risk for
blood clots in blood vessels -- thrombotic disease (TD) -- among dogs
with renal proteinuria -- high levels of protein in urine indicating
kidney damage or disease. They found 150 dogs with renal proteinuria
over a period from 2004 to 2021 in their database. Of those, 50 also had
TD. Five of those 50 dogs were cavalier King Charles spaniels (10%),
which indicated that CKCSs had higher rates of TD among all breeds
diagnosed with renal proteinuria. All five cavaliers had blood clots in
their arteries -- arterial thrombosis (AT) -- four of which were in the
aorta. They suggested, without evidence but based upon prior published
studies, that:
In
a
December 2023 article, Royal Veterinary College (RVC) researchers
Luca Fortuna (right) and Harriet M. Syme studied the risk for
blood clots in blood vessels -- thrombotic disease (TD) -- among dogs
with renal proteinuria -- high levels of protein in urine indicating
kidney damage or disease. They found 150 dogs with renal proteinuria
over a period from 2004 to 2021 in their database. Of those, 50 also had
TD. Five of those 50 dogs were cavalier King Charles spaniels (10%),
which indicated that CKCSs had higher rates of TD among all breeds
diagnosed with renal proteinuria. All five cavaliers had blood clots in
their arteries -- arterial thrombosis (AT) -- four of which were in the
aorta. They suggested, without evidence but based upon prior published
studies, that:
"Reasons suggested for a genetic predisposition of CKCS for TD include a connective tissue disorder, a subset of the breed having increased platelet reactivity, and their increased incidence of cardiac disease. MMVD [myxomatous mitral valve disease] was present in 4 out of 5 of the CKCS with TD and was a common comorbidity in dogs of other breeds with TD in this study; however, ... MMVD is not considered a risk factor for TD."
September 2022:
Acute kidney injury may be a common side effect following
cardiac-bypass surgeries in MVD-affected dogs.
 In
a
September 2022 article, a team of investigators at the Royal
Veterinary College (Daria Starybrat, Rosanne Jepson, Poppy Bristow,
Sarah Peterson, Maha Yerramilli, Murthy Yerramilli, Yu-Mei Chang,
Stefano Cortellini [right]) report finding acute kidney injury
(AKI) in 3 of 19 dogs (15.8%) which were in recovery following cardiac
surgery under cardiopulmonary bypass (CPB). Of the 19 dogs, 7 were
cavalier King Charles spaniels (37%), although the report does not
indicate which dogs developed AKI. Also, two of the dogs died during the
post-operative period prior to discharge from the hospital. They report
finding that specific kidney biomarkers -- inosine and urinary cystatin
B (uCysB) -- changed significantly following the surgeries, potentially
indicating tubular injury of the kidneys. They determined that
MVD-affected dogs undergoing surgeries under CPB are "at increased risk
of AKI", and that biomarkers may promptly identify AKI in order to
implement preventative and theraputic treatments.
In
a
September 2022 article, a team of investigators at the Royal
Veterinary College (Daria Starybrat, Rosanne Jepson, Poppy Bristow,
Sarah Peterson, Maha Yerramilli, Murthy Yerramilli, Yu-Mei Chang,
Stefano Cortellini [right]) report finding acute kidney injury
(AKI) in 3 of 19 dogs (15.8%) which were in recovery following cardiac
surgery under cardiopulmonary bypass (CPB). Of the 19 dogs, 7 were
cavalier King Charles spaniels (37%), although the report does not
indicate which dogs developed AKI. Also, two of the dogs died during the
post-operative period prior to discharge from the hospital. They report
finding that specific kidney biomarkers -- inosine and urinary cystatin
B (uCysB) -- changed significantly following the surgeries, potentially
indicating tubular injury of the kidneys. They determined that
MVD-affected dogs undergoing surgeries under CPB are "at increased risk
of AKI", and that biomarkers may promptly identify AKI in order to
implement preventative and theraputic treatments.
July 2022:
Nine healthy dogs fed only Hill's Prescription Diet h/d dry food
developed steady activation of their renin-angiotensin-aldosterone
system (RAAS).
 In
a
July 2022 article, Iowa State Univ. researchers (Samantha Sotillo,
Jessica L. Ward [right], Emilie Guillot, Oliver Domenig,
Lingnan Yuan, Joseph S. Smith, Vojtech Gabriel, Chelsea A.
Iennarella-Servantez, Jonathan P. Mochel) fed nine healthy dogs a
low-sodium diet of Hill's Prescription Diet h/d dry food for five days.
Their levels of sodium reached such low levels that it resulted in steady activiation of the dogs'
renin-angiotensin-aldosterone system (RAAS). The researchers
intentionally wanted to activate the RAAS in order to conduct a study of
dosages of benazepril, an angiotensin converting enzyme inhibitor
(ACE-I).
In
a
July 2022 article, Iowa State Univ. researchers (Samantha Sotillo,
Jessica L. Ward [right], Emilie Guillot, Oliver Domenig,
Lingnan Yuan, Joseph S. Smith, Vojtech Gabriel, Chelsea A.
Iennarella-Servantez, Jonathan P. Mochel) fed nine healthy dogs a
low-sodium diet of Hill's Prescription Diet h/d dry food for five days.
Their levels of sodium reached such low levels that it resulted in steady activiation of the dogs'
renin-angiotensin-aldosterone system (RAAS). The researchers
intentionally wanted to activate the RAAS in order to conduct a study of
dosages of benazepril, an angiotensin converting enzyme inhibitor
(ACE-I).
EDITOR'S NOTE: Excessively low sodium levels is an electrolyte disorder called hyponatremia.
May 2022:
Pet store cavalier puppy dies of congenital renal dysplasia
despite intensified veterinary care.
 In a
May 2022 article, Michigan State University emergency veterinary
specialists Nicole V. Tusa (right) and Nyssa A. Levy report a case of an
8-week-old intact male cavalier King Charles spaniel diagnosed with
congenital bilateral renal dysplasia. The puppy's main symptoms was
vomiting along with foul smelling breath and being unwilling to eat. He
had been obtained from a pet store three days earlier, and his past
medical history included a surgically corrected umbilical hernia and
intestinal parasites treated with a dewormer, and he had been
vaccinated for distemper and parvovirus. Lab work showed azotemia
(very high levels of nitrogen-containing compounds in the blood),
hyperphosphatemia (very high phosphate level in the blood), and
panhypoproteinemia (very low levels of protein in the blood). Despite
intensived treatment with dialysis for twelve days, the puppy continued
to vomit and decline, ultimately dying of cardiopulmonary arrest. On
post-mortem examination, evidence of bilateral congenital renal
dysplasia was found. The investigators conclude:
In a
May 2022 article, Michigan State University emergency veterinary
specialists Nicole V. Tusa (right) and Nyssa A. Levy report a case of an
8-week-old intact male cavalier King Charles spaniel diagnosed with
congenital bilateral renal dysplasia. The puppy's main symptoms was
vomiting along with foul smelling breath and being unwilling to eat. He
had been obtained from a pet store three days earlier, and his past
medical history included a surgically corrected umbilical hernia and
intestinal parasites treated with a dewormer, and he had been
vaccinated for distemper and parvovirus. Lab work showed azotemia
(very high levels of nitrogen-containing compounds in the blood),
hyperphosphatemia (very high phosphate level in the blood), and
panhypoproteinemia (very low levels of protein in the blood). Despite
intensived treatment with dialysis for twelve days, the puppy continued
to vomit and decline, ultimately dying of cardiopulmonary arrest. On
post-mortem examination, evidence of bilateral congenital renal
dysplasia was found. The investigators conclude:
"Congenital bilateral renal dysplasia should be considered as a differential for azotemia [vomiting] in Cavalier King Charles Spaniel breed. It is hoped that this report will provide an example, as well as raise awareness, for future Cavalier King Charles Spaniel puppies presenting with similar clinical signs and diagnostic findings."
 EDITOR'S NOTE: Oddly, the authors of this article report that:
EDITOR'S NOTE: Oddly, the authors of this article report that:
"While congenital renal dysplasia has been reported in other canine breeds, to the authors' knowledge, this is the first report of congenital renal dysplasia in a CKCS."
Considering that we long have listed five veterinary journal articles, dating as early as 1989, in which cavaliers have been diagnosed with congenital renal dysplasia, it appears that these researchers have not done a thorough job of their research. Indeed, this disorder has been reported as being more prevalent in the cavalier than in the average dog.
April 2022:
Cavaliers are second most common breed in a 132 dog study of
acute kidney injuries.
 In
an
April 2022 article, Israeli researchers Mali Bar-Nathan, Hilla Chen,
Dar Rimer, Gilad Segev (right) studied the long term outcomes
of 132 dogs recovering from acute kidney injuries, focusing on
predictors for the normalization of serum creatinine concentration
(sCr). Cavalier King Charles spaniels were the second most common breed
(5 dogs, 4%), behind German shepherds (9 dogs, 7%). Mixed breeds were
the most common of all (61 dogs, 46%). Higher levels of sCr indicate a
lower flow rate of fluids through the kidneys and a decreased capability
of the kidneys to excrete waste products. Normalization of sCr was 55%
of the dogs at discharge from the treatment facilities and an additional
20% during the follow-up period. The proportion of dogs with sCr
normalization decreased with increase in the grade of kidney injury.
They found that long-term survival was not associated with sCr
normalization, but that the cause of the kidney injury (etiology) was
associated with the long-term outcome. They concluded that long-term
survival of dogs with AKI is longer than previously had been described,
and that normalization of sCr in 99 dogs (75%) occurred, either at
discharge or within the follow-up period. However, normalization of sCr
was not associated with long-term survival. They point out that
"etiology is an important factor determining sCr normalization and
long-term survival, emphasizing the importance of the reversibility of
renal injury rather than its severity."
In
an
April 2022 article, Israeli researchers Mali Bar-Nathan, Hilla Chen,
Dar Rimer, Gilad Segev (right) studied the long term outcomes
of 132 dogs recovering from acute kidney injuries, focusing on
predictors for the normalization of serum creatinine concentration
(sCr). Cavalier King Charles spaniels were the second most common breed
(5 dogs, 4%), behind German shepherds (9 dogs, 7%). Mixed breeds were
the most common of all (61 dogs, 46%). Higher levels of sCr indicate a
lower flow rate of fluids through the kidneys and a decreased capability
of the kidneys to excrete waste products. Normalization of sCr was 55%
of the dogs at discharge from the treatment facilities and an additional
20% during the follow-up period. The proportion of dogs with sCr
normalization decreased with increase in the grade of kidney injury.
They found that long-term survival was not associated with sCr
normalization, but that the cause of the kidney injury (etiology) was
associated with the long-term outcome. They concluded that long-term
survival of dogs with AKI is longer than previously had been described,
and that normalization of sCr in 99 dogs (75%) occurred, either at
discharge or within the follow-up period. However, normalization of sCr
was not associated with long-term survival. They point out that
"etiology is an important factor determining sCr normalization and
long-term survival, emphasizing the importance of the reversibility of
renal injury rather than its severity."
January 2022:
Cavaliers have highest prevalence of renal dysfunction in
Japanese medical records review.
 In
a
January 2022 article, Japanese veterinary researchers (Akiko Uemura,
Lina Hamabe, Ryou Tanaka [right], Noriko Tanaka, Tsuyoshi
Takizawa, Toshiro Iwasaki) examined the blood biochemistry test results
data of 3,347 dogs tested in Japan between 2008 and 2016 to analyze high
and low serum creatinine levels, based upon breed, age, and gender. They
found that, overall, 243 dogs (7.3%) had creatinine over 1.4 mg/dL in
more than one blood test, indicating renal dysfunction. They report that
cavalier King Charles spaniels (64 total dogs) and Shetland sheepdogs
(78 total dogs) had the highest rate of high creatinine levels and
therefore renal dysfunction, at the rate of 14.1%, nearly double the
overall percentage. The researchers speculate that "This may be
connected to the fact that this breed is at high risk of mitral
insufficiency."
In
a
January 2022 article, Japanese veterinary researchers (Akiko Uemura,
Lina Hamabe, Ryou Tanaka [right], Noriko Tanaka, Tsuyoshi
Takizawa, Toshiro Iwasaki) examined the blood biochemistry test results
data of 3,347 dogs tested in Japan between 2008 and 2016 to analyze high
and low serum creatinine levels, based upon breed, age, and gender. They
found that, overall, 243 dogs (7.3%) had creatinine over 1.4 mg/dL in
more than one blood test, indicating renal dysfunction. They report that
cavalier King Charles spaniels (64 total dogs) and Shetland sheepdogs
(78 total dogs) had the highest rate of high creatinine levels and
therefore renal dysfunction, at the rate of 14.1%, nearly double the
overall percentage. The researchers speculate that "This may be
connected to the fact that this breed is at high risk of mitral
insufficiency."
June 2021:
Chronic kidney disease contributes to MVD progression in Korean
study of 63 dogs.
 In
a
June 2021 abstract, a team of Korean researchers (Jeong-Sook Oh,
Dohee Lee, Taesik Yun, Yoonhoi Koo, Yeon Chae, Dongwoo Chang,
Byeong-Teck Kang, Mhan-Pyo Yang, Hakhyun Kim [right]) sought to
evaluate chronic kidney disease (CKD) as a risk factor for the
progression of mitral valve disease (MVD). They reviewed the medical
records of 63 dogs diagnosed with MVD, 44 of which had only MVD and 19
of which had both MVD and CKD. They compared the change in mitral
intensity, vertebral heart scale (VHS), left atrium/aorta ration
(La/Ao), and left ventricle internal diameter (LVIDdN) at the time of
diagnosis with those after 6 months. They report finding that, in MVD
Stages B2 and C, change in the VHS was significantly greater in the
concurrent group than in the MVD group. In all stages of MVD, the
concurrent group showed a greater change in LVIDdN than the MVD group.
However, no significant differences were found in the progression of
murmur grade and left atrium/aorta ratio between the two groups. The
mortality of the concurrent group was significantly higher than that of
the MVD group. They concluded that the results suggest the potential
role of CKD as a risk factor for the progression of MVD.
In
a
June 2021 abstract, a team of Korean researchers (Jeong-Sook Oh,
Dohee Lee, Taesik Yun, Yoonhoi Koo, Yeon Chae, Dongwoo Chang,
Byeong-Teck Kang, Mhan-Pyo Yang, Hakhyun Kim [right]) sought to
evaluate chronic kidney disease (CKD) as a risk factor for the
progression of mitral valve disease (MVD). They reviewed the medical
records of 63 dogs diagnosed with MVD, 44 of which had only MVD and 19
of which had both MVD and CKD. They compared the change in mitral
intensity, vertebral heart scale (VHS), left atrium/aorta ration
(La/Ao), and left ventricle internal diameter (LVIDdN) at the time of
diagnosis with those after 6 months. They report finding that, in MVD
Stages B2 and C, change in the VHS was significantly greater in the
concurrent group than in the MVD group. In all stages of MVD, the
concurrent group showed a greater change in LVIDdN than the MVD group.
However, no significant differences were found in the progression of
murmur grade and left atrium/aorta ratio between the two groups. The
mortality of the concurrent group was significantly higher than that of
the MVD group. They concluded that the results suggest the potential
role of CKD as a risk factor for the progression of MVD.
September 2020:
Kidney disease researchers show their confusion about the 2019
ACVIM Consensus Guidelines' definitions of MVD Stages B1 and B2.
 In a
September 2020 article, a team of Italian and Swiss researchers
(Carlotta Valente [right], Carlo Guglielmini, Oriol Domenech, Barbara Contiero,
Eric Zini, Helen Poser) attempt to determine if symmetric
dimethylarginine (SDMA) concentration, a serum biomarker of renal damage
in dogs, increases as mitral valve disease (MVD) progresses from Stage
B1 to Stages C and D in 78 MVD-affected dogs (including three
cavaliers). They claim to have categorized the MVD-affected dogs
according to the
2019 ACVIM definitions of Stages B1 and B2. However,
they misstate those definitions, and so it is not possible to determine
whether any of the dogs placed in Stage B2 met the 2019 ACVIM version or
not. The 2019 ACVIM Stage B2 requires that both the left atrium and left
ventricle echocardiographic measurements meet or exceed a specific
minimum cut point. In this study, dogs were placed in Stage B2 if either
the left atrium or left ventricle met its minimum cut point. The
researchers conclude:
In a
September 2020 article, a team of Italian and Swiss researchers
(Carlotta Valente [right], Carlo Guglielmini, Oriol Domenech, Barbara Contiero,
Eric Zini, Helen Poser) attempt to determine if symmetric
dimethylarginine (SDMA) concentration, a serum biomarker of renal damage
in dogs, increases as mitral valve disease (MVD) progresses from Stage
B1 to Stages C and D in 78 MVD-affected dogs (including three
cavaliers). They claim to have categorized the MVD-affected dogs
according to the
2019 ACVIM definitions of Stages B1 and B2. However,
they misstate those definitions, and so it is not possible to determine
whether any of the dogs placed in Stage B2 met the 2019 ACVIM version or
not. The 2019 ACVIM Stage B2 requires that both the left atrium and left
ventricle echocardiographic measurements meet or exceed a specific
minimum cut point. In this study, dogs were placed in Stage B2 if either
the left atrium or left ventricle met its minimum cut point. The
researchers conclude:
"In conclusion, the results of this study failed to demonstrate that renal function, evaluated by measuring serum SDMA concentration, is significantly impaired in dogs with MMVD. Although some dogs in the ACVIM Stages] C+D group of MMVD had an increased concentration of the variables used to identify renal dysfunction, this was most likely due to pre-renal azotemia instead of representing a feature of the CRS [cardiorenal syndrome] described in humans."
July 2018:
Spanish researchers find that saliva samples of urea and
creatine can detect chronic kidney disease as well as blood serum
samples.
 In
a
July 2018 article, a team of Spanish researchers (Asta
Tvarijonaviciute, Luis Pardo-Marin, Fernando Tecles, Juana Dolores
Carrillo, Juan Diego Garcia-Martinez, Luis Bernal, Josep Pastor, Jose J.
Cerón [right], Silvia Martinez-Subiela) report finding that
salivary urea and creatinine concentrations were significantly higher in
19 dogs affected with chronic kidney disease (CKD) compared with 17
healthy dogs. They found that urea and creatinine concentrations can be
measured in canine saliva with commercially available spectrophotometric
assays. Both assays showed higher values in saliva of dogs with CKD
compared with healthy dogs and their values were highly correlated with
those in serum.
In
a
July 2018 article, a team of Spanish researchers (Asta
Tvarijonaviciute, Luis Pardo-Marin, Fernando Tecles, Juana Dolores
Carrillo, Juan Diego Garcia-Martinez, Luis Bernal, Josep Pastor, Jose J.
Cerón [right], Silvia Martinez-Subiela) report finding that
salivary urea and creatinine concentrations were significantly higher in
19 dogs affected with chronic kidney disease (CKD) compared with 17
healthy dogs. They found that urea and creatinine concentrations can be
measured in canine saliva with commercially available spectrophotometric
assays. Both assays showed higher values in saliva of dogs with CKD
compared with healthy dogs and their values were highly correlated with
those in serum.
December 2017:
An ACE gene polymorphism may explain why
ACE-inhibitors are less effective in MVD-affected cavaliers.
 In a
December 2017 article, a team of veterinary cardiologists (Meurs KM,
Olsen LH, Reimann MJ, Keene BW, Atkins CE, Adin D, Aona B, Condit J,
DeFrancesco T, Reina-Doreste Y, Stern JA, Tou S, Ward J, Woodruff K)
studied 73 cavalier King Charles spaniels and report finding that:
In a
December 2017 article, a team of veterinary cardiologists (Meurs KM,
Olsen LH, Reimann MJ, Keene BW, Atkins CE, Adin D, Aona B, Condit J,
DeFrancesco T, Reina-Doreste Y, Stern JA, Tou S, Ward J, Woodruff K)
studied 73 cavalier King Charles spaniels and report finding that:
"The CKCS appears to have a high prevalence of the ACE variant. Dogs with the ACE variant had lower levels of ACE activity even in more advanced mitral valve disease than dogs without the variant."
They conclude with the hedge comment that the impact of their finding "on the need for ACE-I in dogs with the polymorphism and heart disease deserves further study."
EDITOR'S NOTE: This is one of four reports by members of this team of researchers regarding the reasons for ACE-inhibitors not being necessary to reduce angiotensin converting enzyme (ACE) activity in treating cavaliers with MVD. We report on two of the others here: this November 2017 article and this June 2016 article. In 2002, the SVEP Study of 229 cavaliers concluded that ACE-inhibitors were ineffective in delaying the onset of heart failure in MVD-affected CKCSs which were in either Stage B1 or B2. These more recent studies help to explain the reasoning behind the SVEP Study findings, which is that ACE-inhibitors do not benefit cavaliers because the breed has reduced ACE activity to begin with.
At some point we would hope that the estimated 70% of veterinary cardiologists who have been ignoring the SVEP Study for the past 15 years and still insist upon prescribing unnecessary ACE-inhibitors to cavaliers might bother to read this body of research and stop treating cavaliers as if they are no different from other breeds with MVD. What is there about the SVEP Study -- the largest veterinary cardiology trial of CKCSs in history -- that these cardiologists cannot understand? When it comes to MVD, the cavalier is a very unique breed, and it is time for all cardiologists to realize that fact and start treating cavaliers accordingly.
October 2017:
USA cardiologists find cavaliers with variant of the
angiotensin-converting enzyme (ACE) gene have lower ACE activity
reaction to mitral valve disease.
 In an
October 2017 article, a team of
mostly USA cardiologists (Kathryn M. Meurs [right], Joshua A. Stern, Clarke E.
Atkins, Darcy Adin, Brent Aona, Julia Condit, Teresa DeFrancesco, Yamir
Reina-Doreste, Bruce W. Keene, Sandy Tou, Jessica Ward, Kathleen
Woodruff) report the results of a study of angiotensin-converting enzyme
(ACE) activity in dogs with mitral valve disease. Of 31 MVD-affected
dogs, including 10 cavalier King Charles spaniels, 11 dogs (6 CKCSs)
were found to have a variant (single nucleotide polymorphism) of the
intron 16 gene, which is associated with ACE activity in MVD-affected
dogs. The ACE polymorphism was most common in the cavalier. They
found that, before any ACE-inhibitor treatment, median ACE activity was
significantly lower for ACE polymorphism dogs than in the control group.
However, following treatment with enalapril, ACE activity was
significantly reduced in both groups. They conclude:
In an
October 2017 article, a team of
mostly USA cardiologists (Kathryn M. Meurs [right], Joshua A. Stern, Clarke E.
Atkins, Darcy Adin, Brent Aona, Julia Condit, Teresa DeFrancesco, Yamir
Reina-Doreste, Bruce W. Keene, Sandy Tou, Jessica Ward, Kathleen
Woodruff) report the results of a study of angiotensin-converting enzyme
(ACE) activity in dogs with mitral valve disease. Of 31 MVD-affected
dogs, including 10 cavalier King Charles spaniels, 11 dogs (6 CKCSs)
were found to have a variant (single nucleotide polymorphism) of the
intron 16 gene, which is associated with ACE activity in MVD-affected
dogs. The ACE polymorphism was most common in the cavalier. They
found that, before any ACE-inhibitor treatment, median ACE activity was
significantly lower for ACE polymorphism dogs than in the control group.
However, following treatment with enalapril, ACE activity was
significantly reduced in both groups. They conclude:
"An ACE polymorphism is associated with lower levels of ACE activity. Dogs with the polymorphism still experience suppression of ACE activity in response to an ACE inhibitor. It is possible that the genetic status and ACE activity of dogs may impact the response of dogs with this variant to an ACE inhibitor."
EDITOR'S NOTE: We already know from the SVEP study of 229 CKCSs and the VETPROOF trial that ACE-inhibitors are ineffective in treating MVD-affected cavaliers prior to heart failure. This study suggests a reason for those previous findings. Perhaps cavaliers tend to have a lower level of ACE activity in reaction to their mitral valve disease, and so treating with ACE-inhibitors is not as necessary to keep the angiotensin-converting enzyme activity under control.
October 2016: Korean
researchers find cystatin-C or cystatin 3 (Cys-C) and symmetric
dimethylarginine (SDMA) as useful biomarkers for canine kidney disease.
 In
a
September 2016 study by a team of Korean cardiology researchers
(Bum-Sul Choi, Hyeong-Sun Moon, Sang-Hyuk Seo, Changbaig Hyun
[right]), they examined serum concentration levels of two kidney
function biomarkers -- Cystatin-C or cystatin 3 (Cys-C) and symmetric
dimethylarginine (SDMA). They confirmed the findings of previous studies
involving SDMA, noting that SDMA does not appear to be influenced by
age, body weight or gender. Regarding Cys-C, they disagreed with prior
studies findings that the Cys-C concentration was influenced by age and
body weight. They stated, "However, our study strongly suggested that
the Cys-C was not influenced by age or body weight." Nevertheless, they
concluded that, "Although the Cys-C is a promising renal marker for
canine kidney disease, test standardization and reference range have yet
been clearly established in dogs."
In
a
September 2016 study by a team of Korean cardiology researchers
(Bum-Sul Choi, Hyeong-Sun Moon, Sang-Hyuk Seo, Changbaig Hyun
[right]), they examined serum concentration levels of two kidney
function biomarkers -- Cystatin-C or cystatin 3 (Cys-C) and symmetric
dimethylarginine (SDMA). They confirmed the findings of previous studies
involving SDMA, noting that SDMA does not appear to be influenced by
age, body weight or gender. Regarding Cys-C, they disagreed with prior
studies findings that the Cys-C concentration was influenced by age and
body weight. They stated, "However, our study strongly suggested that
the Cys-C was not influenced by age or body weight." Nevertheless, they
concluded that, "Although the Cys-C is a promising renal marker for
canine kidney disease, test standardization and reference range have yet
been clearly established in dogs."
January 2016: Renal lesions were found in 52.2% of post-mortem samples of cavaliers in UK study. In an April 2016 article, UK researchers (Kent, Andrew C. C.; Constantino-Casas, Fernando; Rusbridge, Clare; Corcoran, Brendan; Carter, Margaret; Ledger, Tania; Watson, Penny J.) searched for pancreatic, hepatic (liver) and renal (kidney) lesions in post-mortem samples from 54 cavalier King Charles spaniels (CKCSs). The rate of diagnosis of renal disease prior to death was in 16.7% of the CKCSs, but the post-mortem examination found evidence of renal lesions in 52.2% of the dogs. The researchers concluded that renal lesions are common in the breed, and that clinicians should be aware of this when presented with clinical cases.
September 2015:
Cavalier is diagnosed with rare "rubber jaw" due to end-stage
renal failure.
 In
a
September 2015 article by New Zealand clinician Judith Visser
(right), she reports a case of a 5-year-old female cavalier King
Charles spaniel suffering from anorexia (inability to eat because of
difficulty picking up, chewing, or swallowing food). The dog had
flexibility of the jaw and instability of the teeth. X-rays showed
severely reduced bone density, and blood work showed a markedly elevated
phosphorous level, making primary hyperparathyroidism unlikely. The
author tentatively diagnosed "end-stage renal failure with secondary
renal hyperparathyroidism waas made based on the presence of marked
azotemia, hyperphos-phatemia and osteopenia." Necropsy showed the
mandibles, maxillae and ribs were severely pliable and easy to bend.
Multifocal calcium deposits were present on the parietal pleura (the
lining of the chest cavity that contains the lungs), the kidneys were
small and pale, and the parathyroid glands were severely enlarged. She
diagnosed chronic end-stage renal failure with severe metastatic
calcification and fibrous osteodystrophy was made.
In
a
September 2015 article by New Zealand clinician Judith Visser
(right), she reports a case of a 5-year-old female cavalier King
Charles spaniel suffering from anorexia (inability to eat because of
difficulty picking up, chewing, or swallowing food). The dog had
flexibility of the jaw and instability of the teeth. X-rays showed
severely reduced bone density, and blood work showed a markedly elevated
phosphorous level, making primary hyperparathyroidism unlikely. The
author tentatively diagnosed "end-stage renal failure with secondary
renal hyperparathyroidism waas made based on the presence of marked
azotemia, hyperphos-phatemia and osteopenia." Necropsy showed the
mandibles, maxillae and ribs were severely pliable and easy to bend.
Multifocal calcium deposits were present on the parietal pleura (the
lining of the chest cavity that contains the lungs), the kidneys were
small and pale, and the parathyroid glands were severely enlarged. She
diagnosed chronic end-stage renal failure with severe metastatic
calcification and fibrous osteodystrophy was made.
September 2015: International team of cardiologists and nephrologists issue a Consensus Statement on cardio-renal disorders. In a September 2015 report, a team of 9 veterinary cardiologists and 7 veterinary nephrologists from Europe and North America (J. L. Pouchelon, C. E. Atkins, C. Bussadori, M. A. Oyama, S. L. Vaden, J. D. Bonagura, V. Chetboul, L. D. Cowgill, J. Elliot, T. Francey, G. F. Grauer, V. Luis Fuentes, N. Sydney Moise, D. J. Polzin, A. M. Van Dongen, N. Van Israël) have issued a "Consensus Statement" to increase the awareness of and codify the definition, classification, diagnosis, and management strategies for veterinary patients with cardio-renal syndrome (CRS), with an emphasis on the pathological interplay between the two organ systems. They acknowledge "a growing understanding of the complexity of interplay between renal and cardiovascular systems in both health and disease."
 They
have observed: "The concept of CRS, which involves a bidirectional
pathway of injury wherein disease of either organ directly or indirectly
contributes to injury of the other." And they have included a chart
showing postulated mechanisms underlying the relationship between heart
failure (HF) and renal dysfunction. (Click on the thumbnail chart at
right.)
They
have observed: "The concept of CRS, which involves a bidirectional
pathway of injury wherein disease of either organ directly or indirectly
contributes to injury of the other." And they have included a chart
showing postulated mechanisms underlying the relationship between heart
failure (HF) and renal dysfunction. (Click on the thumbnail chart at
right.)
Among their consensus statements, are:
"Statement 8: Thoracic radiography is recommended to assess the presence or absence of congestive heart failure, and echocardiography is recommended to assess cardiac morphology, lesions, and to estimate relevant haemodynamic parameters.
"Statement 9: Renal imaging is recommended to improve diagnosis, prognosis and guide potential therapies in CvRD. Conventional abdominal radiographs and ultrasound are recommended to help detect morphological abnormalities and determine underlying aetiology.
"Statement 10: As the kidney and heart are two organs at risk for damage due to systemic hypertension, and as kidney disease is often associated with systemic arterial hypertension, systemic arterial blood pressure should be systematically monitored in both kidney and cardiovascular diseases. * * *
"Statement 13: ... particular attention should be directed towards the following when managing any form of [CRS]: 1) identification and treatment of elevated blood pressure as per IRIS recommendations; 2) stepwise titration of dosages of diuretics, ACEI, inotropes and/or fluids with frequent monitoring of renal function, body weight, hydration, electrolyte status, and systemic blood pressure (i.e. performed and rechecked within 3-5 days following initiation or dose adjustment of these drugs); 3) proper nutrition, with respect to reduced dietary sodium and phosphate and appropriate protein and caloric intake."
 July 2015:
Swedish vet student reports the cavalier has a high incidence of kidney
disease in Sweden. In a
July 2015 graduate thesis, a
Swedish veterinary student, Lynn Hellstrom (right), reviewed veterinary records
of insured dogs in Sweden diagnosed with kidney diseases. She reports
that the cavalier King Charles spaniel and the Shetland sheepdog had the
highest incidence of kidney disease, while noting that "Neither one of
these breeds are commonly mentioned as particuladysplairly affected by kidney
diseases in the literature of veterinary medicine." She could find "no
specific etiology of kidney disease ... diagnosed more often than others
in ... CKCS dogs. Renal dysplasia was diagnosed in dogs of both breeds."
July 2015:
Swedish vet student reports the cavalier has a high incidence of kidney
disease in Sweden. In a
July 2015 graduate thesis, a
Swedish veterinary student, Lynn Hellstrom (right), reviewed veterinary records
of insured dogs in Sweden diagnosed with kidney diseases. She reports
that the cavalier King Charles spaniel and the Shetland sheepdog had the
highest incidence of kidney disease, while noting that "Neither one of
these breeds are commonly mentioned as particuladysplairly affected by kidney
diseases in the literature of veterinary medicine." She could find "no
specific etiology of kidney disease ... diagnosed more often than others
in ... CKCS dogs. Renal dysplasia was diagnosed in dogs of both breeds."
January 2015:
Idexx Labs announces new early test -- SDMA -- for chronic kidney
disease.
![]() In a
January 2015 report by Dr. Jane Robertson, Idexx Laboratories has
announced a new biomarker test for CKD, called symmetric
dimethyl-arginine (SDMA), which reportedly will enable diagnosis of CKD
several months or even years earlier than creatinine tests. SDMA is a
methylated form of the amino acid arginine, which is released into the
circulation during protein degradation and is excreted almost
exclusively by the kidneys. SDMA reportedly increases earlier than
creatinine in CKD; it increases on average with 40% loss of kidney
function versus creatinine, which does not increase until 75% of kidney
function is lost.
In a
January 2015 report by Dr. Jane Robertson, Idexx Laboratories has
announced a new biomarker test for CKD, called symmetric
dimethyl-arginine (SDMA), which reportedly will enable diagnosis of CKD
several months or even years earlier than creatinine tests. SDMA is a
methylated form of the amino acid arginine, which is released into the
circulation during protein degradation and is excreted almost
exclusively by the kidneys. SDMA reportedly increases earlier than
creatinine in CKD; it increases on average with 40% loss of kidney
function versus creatinine, which does not increase until 75% of kidney
function is lost.
Idexx states that it expects to begin trials with several hundred North American veterinary clinic customers by the end of March 2015. Following the conclusion of those trials, Idexx states that it will include the SDMA test as part of the standard chemistry panel in North America in the summer of 2015.
May 2013:
UK study finds cavaliers at "significant risk" for chronic kidney disease.
In a
July
 2013 study of 107,214 dogs treated in 2010 and 2011 at 89 UK
veterinary clinics, the Royal Veterinary College researchers found that cavalier King Charles
spaniels and Cocker spaniels were a "significant risk factor" for developing
chronic kidney disease (CKD). They also reported that cardiac disease was a
significant co-disease with CKD.
2013 study of 107,214 dogs treated in 2010 and 2011 at 89 UK
veterinary clinics, the Royal Veterinary College researchers found that cavalier King Charles
spaniels and Cocker spaniels were a "significant risk factor" for developing
chronic kidney disease (CKD). They also reported that cardiac disease was a
significant co-disease with CKD.
Key findings from the study included:
• Overall canine CKD prevalence
0.21%.
• Dogs aged over 12 years had 5
times the odds of kidney disease, compared with dogs aged between 7 and 12
years.
• Predisposed breeds included
the cavalier and Cocker spaniel.
• The median survival time after
CKD diagnosis was 266 days.
• IRIS staging and blood urea
analysis values at diagnosis were useful predictors of survival.
• The study shows the power of
primary practice data to better understand disorders seen in primary practice.
The study concluded that chronic kidney disease compromises dog welfare. Increased awareness of CKD risk factors and association of blood biochemistry results with survival time should facilitate diagnosis and optimize case management to improve animal survival and welfare.
Professor Jonathan Elliott, one of the co-authors, commented that:
"This is an exciting paper that provides valuable information for clinicians in relation to the types of dogs that are at increased risk of kidney disease and their likely prognosis following diagnosis. This type of study is only possible because of the extensive database of case records that VetCompass provides. Vets in practice can use this information to improve the evidence on which they can base their advice to owners."
RETURN TO TOP
Related Links
RETURN TO TOP
Veterinary Resources
Xanthine calculi in a dog. Kidder, D. E. & Chivers, P. R. Vet.Rec. 1968;83(9):228-229. [The dog was a cavalier King Charles spaniel.]
The renin-angiotensin-aldosterone system in congestive failure in conscious dogs. L Watkins, Jr, J A Burton, E Haber, J R Cant, F W Smith, A C Barger. J Clin Invest. June 1976;57(6):1606-1617. Quote: "The role of the renin-angiotensin-aldosterone system in the development of congestive failure has been assessed in the conscious dog by use of the nonapeptide converting enzyme inhibitor. Constriction of the pulmonary artery or thoracic inferior vena cava was maintained for 2 wk while daily measurements were made of plasma renin activity, plasma aldosterone, plasma volume, hematocrit, serum sodium and potassium concentrations, sodium and water balance, body weight, and arterial, caval, and atrial pressures. The initial response to constriction was a reduction in blood pressure, a rise in plasma renin activity, plasma aldosterone, and water intake, and nearly complete sodium retention. In the days after moderate constriction plasma volume and body weight increased (with development of ascites and edema); blood pressure, sodium excretion, plasma renin acvitity, and plasma aldosterone returned to normal. In animals in which blood pressure was not restored, plasma renin activity and plasma aldosterone remained elevated throughout the period of constriction. Single injections of converting enzyme inhibitor reduced blood pressure when plasma renin activity was elevated. Chronic infusion of the inhibitor in dogs with thoracic inferior vena caval constriction prevented the restoration of blood pressure and suppressed the rise in plasma aldosterone; sodium retention and volume expansion were less than in control experiments. Thus the renin-angiotensin-aldosterone system plays an essential role in the maintenance of blood pressure during the genesis of congestive failure. Initially, the restoration of blood pressure is dependent upon circulating angiotensin II; in the later stages, blood pressure is dependent upon the increase in plasma volume."
Renal Dysplasia in a Cavalier King Charles Spaniel. Murphy, M.G. Irish Veterinary Journal 42:96-97, May 1989. Quote: "A 5-month-old female Cavalier King Charles Spaniel developed polydipsia, polyuria (specific gravity:1.010), vomiting and diarrhoea. Biochemical tests showed elevated blood urea nitrogen (15.05 mmol/litre)."
Effects of Dietary Protein Intake on Renal Functions. Delmar R. Finco. Veterinary Forum. 1999;16(10):34-44. Quote: "Older dogs have a higher incidence of chronic renal disease than young dogs, and restricting protein intake in these dogs has been advocated as a renoprotective maneuver. In a study designed to test this hypothesis, experimental dogs 7 to 8 years of age were divided into two groups. Dogs in both groups had uninephrectomy performed to increase vulnerability of the remaining kidney to any protein effects. One group was fed a low protein diet, and the other group received a high protein diet for the subsequent 4 years. Results of this study indicated that there were no adverse effects of the high protein diet (Table 1), and mortality was actually higher in the low protein group. A similar study was conducted in another laboratory, and, likewise, no adverse effect of high protein diets was detected. In the latter study, however, increased mortality in dogs receiving the low protein diet did not occur."
Mythology of Protein Restriction for Dogs with Reduced Renal Function. Kenneth C. Bovee. Compendium on Continuing Education for the Practicing Veterinarian. Nov. 1999;21(11):15-20. Quote: "Advantages and Disadvantages of Dietary Protein Restriction in Dogs: Based on the previous data, the only advantages appear to be a lowering of BUN and the possibility of reduced nausea. Quantifying the value of these effects has not been reported in dogs. On the contrary, there appear to be disadvantages to reduced protein intake. These include reduced kidney function as measured by GFR and renal plasma flow, possibility of a negative nitrogen balance, and the promotion of a catabolic state in the presence of proteinuria. In practical application the use of a vague dietary recommendation appears to lead to complacency about long-term surveillance of the animal or the need for individualized specific treatment. Because some sort of management appears available, the search for a more specific etiologic diagnosis is usually not mounted. Finally, the use of arbitrary diets leads to a delusion of ourselves and clients about treatment and increases the cost to owners. Why Is Dietary Alteration Still Used if There Is No Proven Benefit?: The continued use of protein restriction in the absence of scientific evidence deserves thoughtful consideration. I would suggest that the dogma and mythology of a possible benefit are so embedded in the thought process of veterinarians and owners that these cannot be easily dislodged despite the scientific evidence. I would refer to this as the myth of dietary protein and characterize it as a negative myth."
Congenital and Inherited Renal Disease of Small Animals. Deborah S. Greco. Vet. Clinics: Sm. Anim. Pract. March 2001; doi: 10.1016/S0195-5616(01)50211-9. Quote: Normal pediatric patients have immature kidneys, impaired renal function, and decreased renal blood flow and glomerular filtration rate until approximately 10 weeks of age. The immaturity of the kidneys leads to clinical implications in the treatment of pediatric veterinary patients, particularly when renal compromise is present. Pediatric patients are more susceptible to dehydration and overhydration. Similarly, drugs eliminated by or toxic to the kidneys should be used cautiously in pediatric patients. Congenital renal diseases are present at birth and may be determined genetically; familial renal disorders occur in related animals with a higher frequency than would be expected by chance, and frequently are inherited. The most common familial disorders in cats and dogs include renal amyloidosis, renal dysplasia, polycystic kidneys, basement membrane disorders, and tubular dysfunction (Fanconi's syndrome). This article alerts the veterinarian to commonly observed congenital and hereditary conditions of the kidneys in small animals. ... Disorganized parenchyma and segmental or focal areas of immature or anomalous structures in an otherwise normal kidney characterize renal dysplasia. Renal aplasia refers to a more severe generalized form of dysplasia that affects the entire kidney. These conditions are observed in male and female puppies of many breeds [including the cavalier King Charles spaniel] and rarely in kittens. Puppies and kittens with renal dysplasia are often clinically normal for extended periods of time before signs of chronic renal failure ensue. The age of onset of clinical signs range from 4 weeks to 5 years; however, most are seen before 2 years of age.
Renal dysplasi hos hund. Anette Wiholm Zander. Swedish Univ. 2003. Quote: The purpose of this study was to obtain more knowledge about epidemiology, breed predisposition and morphology of renal dysplasia. In the epidemiological part of the study the prevalence of renal dyplasia in nine different breeds during the time period 1981-2001 was investigated. In the histopathological part the presence of dysplastic changes in the kidneys was examined in 57 cases of renal dysplasia in Tibetan Spaniel and Flat-coated Retriever. The highest prevalences for the whole time period were noted for Shih Tzu (0,57%), Tibetan Terrier (0,56%), Lhasa Apso (0,48%) and Tibetan Spaniel (0,42%). For the breeds Irish Soft-coated Wheaten Terrier, Cairn Terrier and Flat-coated Retriever the prevalence was a little bit lower (0,29%, 0,26% and 0,20%, respectively) and for English Cocker Spaniel and Cavalier King Charles Spaniel [20 dogs] the prevalence was very low (0,09% and 0,08%, respectively). ... More than half (64,2 %) of the dogs in this study were < 2 years of age at the time of diagnos/euthanasia, and the highest proportion of dogs < 2 years of age was noted for Cairn Terriers, Tibetan Terriers and Lhasa Apsos (80,0%, 80,0% and 75,0%, respectively). A sex predisposition for renal dysplasia was detected for Flat-coated Retrievers and Cavalier King Charles Spaniels, in which females represented 87,5% and 85,0 %, respectively, of the cases. In the morphological study, the most commonly detected signs of renal dysplasia were foetal glomeruli, dysplastic tubules and persistent metanephric ducts in both the Tibetan Spaniel and the Flat-coated Retriever. These lesions were found at a frequency of 89%, 22% and 18% in the Tibetan Spaniel and 80%, 53% and 7% in the Flat-coated Retriever, respectively. The observed difference between the two breeds regarding dysplastic tubules was statistically significant (p=0,016). ... In conclusion, the present study showed that most of the cases of renal dysplasia in the nine breeds examined were diagnosed before the age of two years. ... For the breeds Flat-coated Retriever and Cavalier King Charles Spaniel, the results indicate a sex predisposition for renal dysplasia, with females clearly over-represented.
Renal Dysplasia with Unilateral Renal Agenesis in a Dog. T. Morita, Y. Michimae, M. Sawada, T. Uemura, Y. Araki, A. Haruna, A. Shimada. J. Comparative Pathol. July 2005; doi: 10.1016/j.jcpa.2005.01.002. Quote: This report describes a renal dysplastic lesion associated with renal agenesis in a 3-year-old [cavalier King Charles spaniel] dog with chronic renal failure. Haematological examination revealed non-regenerative anaemia, azotaemia, increased creatinine and hyperphosphataemia. At necropsy, the right kidney and right ureter could not be identified. The left kidney was slightly enlarged, with a reduced cortico-medullary ratio. Histologically, the medulla of the left kidney had persistent mesenchyme and primitive tubules (tall pseudostratified columnar epithelium), dilated collecting ducts lined by flattened epithelium, and adenomatoid proliferation of cuboidal epithelium; fetal or immature glomeruli could not be identified. To our knowledge, this is the first report of a renal dysplastic lesion with unilateral agenesis in animals.
Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. L.G. Pedersen, I. Tarnow, L.H. Olsen, T. Teerlink, H.D. Pedersen. Research in Vet. Sci. 2006;80:336-342. Quote: Asymmetric dimethylarginine (ADMA) is a marker of various cardiovascular diseases in man. The aim of the present study was to test if Cavalier King Charles Spaniels (CKCS) with varying degrees of mitral regurgitation (MR) had increased plasma concentration of ADMA and furthermore, characterize the plasma level of ADMA and symmetric dimethylarginine (SDMA) in normal dogs. Seventy-six dogs were included (44 CKCS and 32 dogs of other breeds). The CKCS had various degrees of MR, whereas the remaining dogs had either no or minimal MR. Apart from cardiac murmurs, no dogs showed signs of cardiac or systematic disease. The degree of MR had no significant influence on ADMA (P = 0.33). Body weight was directly associated with ADMA (P = 0.0004) and creatinine was directly associated with SDMA (P < 0.0001). Furthermore, the plasma concentration of ADMA was three to four times higher than found in healthy humans.
Bilateral renal agenesis in two cavalier King Charles spaniels. Yates, Georgina H; Sanchez-Vazquez, Manuel J; Dunlop, Morag M. Vet. Rec. May 2007; doi: 10.1136/vr.160.19.672-a. Quote: The [cavalier King Charles spaniel] bitch was not on any medication during the pregnancy apart from fenbendazole (Panacur; Intervet) at the licensed dose rate. This was its third pregnancy and there had been no problems during previous pregnancies or litters. ... Bilateral renal agenesis is a very rare finding in dogs, and this case has the additional peculiarity that two puppies from the same litter were presented with the condition.
Pet Food Safety: Dietary Protein. D.P. Laflamme. Topics in Companion Animal Medicine. Aug. 2008;23(3):154-157. Quote: "Based on a comprehensive review, there remains no evidence that dietary protein causes kidney damage, or any other adverse effects, in healthy dogs. Even in dogs with chronic kidney disease, dietary protein does not appear to contribute to kidney damage. However, in chronic kidney disease, there can be an accumulation of byproducts of protein metabolism, which may contribute to uremic signs. Hence, in these patients, dietary protein restriction may be of benefit. On the other hand, dietary protein is important to support normal protein turnover and maintain lean body mass. Healthy, aging dogs have an increased requirement for dietary protein. Vhen insufficient protein is provided, it can aggravate the age-associated loss of lean body mass and may contribute to earlier mortality. Unless medically indicated, intake of dietary protein should not be restricted."
Cardiorenal Syndrome. Claudio Ronco, Mikko Haapio, Andrew A. House, Nagesh Anavekar, Rinaldo Bellomo. J. Amer. College Cardiology. November 2008; doi: 10.1016/j.jacc.2008.07.051. Quote: The term cardiorenal syndrome (CRS) increasingly has been used without a consistent or well-accepted definition. To include the vast array of interrelated derangements, and to stress the bidirectional nature of heart-kidney interactions, we present a new classification of the CRS with 5 subtypes that reflect the pathophysiology, the time-frame, and the nature of concomitant cardiac and renal dysfunction. CRS can be generally defined as a pathophysiologic disorder of the heart and kidneys whereby acute or chronic dysfunction of 1 organ may induce acute or chronic dysfunction of the other. Type 1 CRS reflects an abrupt worsening of cardiac function (e.g., acute cardiogenic shock or decompensated congestive heart failure) leading to acute kidney injury. Type 2 CRS comprises chronic abnormalities in cardiac function (e.g., chronic congestive heart failure) causing progressive chronic kidney disease. Type 3 CRS consists of an abrupt worsening of renal function (e.g., acute kidney ischemia or glomerulonephritis) causing acute cardiac dysfunction (e.g., heart failure, arrhythmia, ischemia). Type 4 CRS describes a state of chronic kidney disease (e.g., chronic glomerular disease) contributing to decreased cardiac function, cardiac hypertrophy, and/or increased risk of adverse cardiovascular events. Type 5 CRS reflects a systemic condition (e.g., sepsis) causing both cardiac and renal dysfunction. Biomarkers can contribute to an early diagnosis of CRS and to a timely therapeutic intervention. The use of this classification can help physicians characterize groups of patients, provides the rationale for specific management strategies, and allows the design of future clinical trials with more accurate selection and stratification of the population under investigation.
99mTc-DTPA diuretic renal scintigraphy in dogs with nephroureterolithiasis. Silke Hecht, S. Meg Lawson, India F. Lane, Dorothy E. Sharp, Gregory B. Daniel. Can Vet J 2010;51:1360-1366. Quote: "This study evaluated the results of diuretic renal scintigraphy in dogs with urolithiasis. Eighty-three kidneys with nephroureterolithiasis 1/2 renal pelvis/ureteral dilation were included in the study [including two cavalier King Charles spaniels]. Sixty-three kidneys showed a non-obstructive pattern, with a steep drop or gradual downward slope of renal time-activity curve (TAC). Excretion half-time of radiopharmaceutical (T1/2) was 3.99 (2.99 to 7.95) min. Three kidneys showed an obstructive pattern, with continuous rise of the TAC and median T1/2 of 210.71 (25.20 to 217.56) min. Fifteen kidneys had non-diagnostic studies characterized by flat TAC. Individual kidney glomerular filtration rate was , 0.5 mL/min/kg body weight in most non-diagnostic studies. Diuretic renal scintigraphy appears to be a useful adjunct modality to rule out or confirm ureteral obstruction in dogs. Additional diagnostic procedures may be necessary to achieve a definitive diagnosis in cases of severely impaired renal function."
Cardio-Renal Syndrome, what is behind it? A rock and a hard place: cardiorenal syndrome in clinical canine veterinary patients. Rebecca L. Stepien. CEVA CardioSymposium, October 2011. Quote: "Cardio-renal syndrome has been variously defined. In clinical medicine, it may be viewed as 'a state In which therapy to relieve heart failure symptoms is limited by worsening renal function', but this arguably is a clinical view reflecting a cardiologist's concern; a nephrologist may be more likely to define CRS as ' ... a normal kldney that is dysfunctional because of a diseased heart ...'. An expansive, multisystem view is required In order to understand the complex bidirectional interactions of these two critical body systems, in which '... each dysfunctional organ has the ability to initiate and perpetuate disease in the other organ through common hemodynamic, neurohormonal, and immunological/ biochemical feedback pathways'. ... Cardio-renal syndrome may be divided into 5 types. Type 2 CRS appears to be of significant clinical concern in cardiac patients. ... The prevalence of azotemia and decreased renal function in canine CHF patients is similarly high. In a report in 2010, 24.1% of 223 dogs with heart disease were azotemic, although the prevalence of CHF in this cohort of heart patients was not specified. In an eartler study, Nicolle and colleagues reported that 50% of small dogs (<13 kg) with degenerative valve disease were azotemic either by Blood Urea Nitrogen (BUN) or Creat or both. This study included dogs in all stages of heart failure (NYHA grades I-IV) and some were treated at the time of the study. As their NYHA class increased, a greater percentage of patients were azotemic and were also more likely to have already been treated with some combination of angiotensin-converting enzyme inhibitors, furosemide, spironolactone and digoxin. Older dogs were also more likely to be azotemic. The severity of CHF was also linked to renal function; Class III-IV dogs had 45% decrease in GFR compared with class I-II dogs, and 7/9 grade III-IV dogs had abnormally decreased GFR. In human and canine patients, the presence and severity of RAAS activation and azotemia is affected by therapy, especially furosemide and vasodilators. ... In some cases, inadequate therapy of venous congestion may contribute to renal dysfunction, resulting in 'congestive kidney failure'. Human and veterinary literature supports the use of ACEI and aldosterone receptor antagonists such as spironolactone to limit the RAAS activation that has beer documented with CHF therapy, and use of these modalities has prolonged survival times in affected patients. The topic of cardiorenal syndrome is becoming an increasingly important concern in clinical veterinary medicine. Avoidance of WRF in dogs before and during CHF therapy is crucial to maintain quality of life as survival in dogs with this common disease increases."
Chronic Kidney Disease in Dogs in UK Veterinary Practices: Prevalence, Risk Factors, and Survival. D.G. O'Neill, J. Elliott, D.B. Church, P.D. McGreevy, P.C. Thomson, and D.C. Brodbelt. J.Vet.Intern.Med. July 2013;27(4):814-821. Quote: "Background: The prevalence for chronic kidney disease (CKD) in dogs varies widely (0.05-3.74%). Identified risk factors include advancing age, specific breeds, small body size, and periodontal disease. Hypothesis/Objectives: To estimate the prevalence and identify risk factors associated with CKD diagnosis and survival in dogs. Purebred dogs were hypothesized to have higher CKD risk and poorer survival characteristics than crossbred dogs. Animals: A merged clinical database of 107,214 dogs attending 89 UK veterinary practices over a 2-year period (January 2010-December 2011). Methods: A longitudinal study design estimated the apparent prevalence (AP) whereas the true prevalence (TP) was estimated using Bayesian analysis. A nested case-control study design evaluated risk factors. Survival analysis used the Kaplan-Meier survival curve method and multivariable Cox proportional hazards regression modeling. Results: The CKD AP was 0.21% (95% CI: 0.19-0.24%) and TP was 0.37% (95% posterior credibility interval 0.02-1.44%). Significant risk factors included increasing age, being insured, and certain breeds (Cocker Spaniel, Cavalier King Charles Spaniel). Cardiac disease was a significant comorbid disorder. Significant clinical signs included halitosis, weight loss, polyuria/polydipsia, urinary incontinence, vomiting, decreased appetite, lethargy, and diarrhea. The median survival time from diagnosis was 226 days (95% CI 112-326 days). International Renal Interest Society stage and blood urea nitrogen concentration at diagnosis were significantly associated with hazard of death due to CKD. Conclusions and Clinical Importance: Chronic kidney disease compromises dog welfare. Increased awareness of CKD risk factors and association of blood biochemistry results with survival time should facilitate diagnosis and optimize case management to improve animal survival and welfare."
Consensus Recommendations for Standard Therapy of Glomerular Disease in Dogs. IRIS Canine GN Study Group Standard Therapy Subgroup, S. Brown, J. Elliott, T. Francey, D. Polzin, S. Vaden. J. Vet. Intern. Med. November 2013; doi: 10.1111/jvim.12230. Quote: Canine glomerular disease is here considered to be primary if the process that initiates renal injury originates in the glomerulus. In most renal diseases, regardless of the site of the initiating renal injury, there are pathologic changes that occur in glomeruli after injury in other compartments (eg, tubulointerstitial disease or generalized loss of nephrons), and these changes are termed secondary glomerular disease. The recommendations outlined below apply to canine glomerular diseases in general, both primary and secondary. In the management of dogs with glomerular disease, some recommendations are standard therapeutic considerations for all dogs with glomerular disease, regardless of the cause or severity. Although the present recommendations are considered a standard part of treatment in all canine glomerular diseases, their institution in a patient requires considerable prudence on the part of the veterinarian and individualized therapeutic plans, with adjustments based on severity of the glomerular disease and other patient factors. The severity of a glomerular disease is usually reflected in the magnitude of proteinuria,1 assessed as the urine protein-to-creatinine ratio (UPC), especially early in the disease process. ... Standard therapy forms the basic foundation for care of dogs with glomerular disease, as it is herein recommended for use in all affected animals regardless of causation of the disease. Consensus recommendations target the evaluation and management of proteinuria, inhibition of the renin-angiotensin-aldosterone system, modification in dietary intake with special consideration for those nutrients with renal effects, diagnosis and treatment of systemic hypertension, and evaluation and management of body fluid volume status in dogs with glomerular disease.
Cardiorenal Syndrome: Cardiologist vs. Nephrologist. Joao S. Orvalho, Larry D. Cowgill. ACVIM Forum. June 2014. Quote: "Introduction: It has become increasingly recognized by cardiologists and nephrologists that there are important bidirectional functional and pathological interactions between the heart and the kidney, wherein dysfunction of either organ promotes clinical worsening of the other.1 Cardiovascular disease constitutes a significant threat for patients with renal disease, and renal dysfunction is also often present in patients with cardiac disease. The clinical consequences of these interactions have gained increasing focus and have prompted further definition, classification, and understanding. These interactions are the pathophysiological basis for the clinical entity termed cardiorenal syndrome (CRS) in human medicine. Cardiorenal syndrome per se has not been well characterized in veterinary medicine. ... Conclusions and Future Directions: An accurate appreciation of the kidney and the cardiovascular system and their interactions may have practical clinical implications. A multidisciplinary evaluation including the expertise of cardiologists and nephrologists may be the most appropriate approach for the patient at risk for CRS.43 The outcome of CRS patients is likely to improve with the increasing awareness and ability to identify and understand the pathophysiological characteristics of cardiorenal syndrome."
Earlier Diagnosis of Kidney Disease: SDMA allows earlier intervention for more effective management of kidney disease. Jane Robertson. Idexx Labs. January 2015. Quote: "Over their lifetime, 1 in 3 cats and 1 in 10 dogs could develop chronic kidney disease (CKD)--a leading cause of death, particularly in cats. Symmetric dimethylarginine (SDMA) is a revolutionary new kidney function test that will enable veterinarians to diagnose CKD in cats and dogs months or even years earlier than traditional methods, making it possible to intervene earlier and more effectively manage kidney disease. SDMA is a methylated form of the amino acid arginine, which is produced in every cell and released into the body's circulation during protein degradation. SDMA is excreted almost exclusively by the kidneys, making it a good marker for estimating kidney function. Research has shown that SDMA can identify CKD an average of 9 months earlier in dogs and 17 months sooner in cats--in one cat 4 years earlier. In addition, SDMA is not impacted by muscle mass, thereby providing practitioners a better tool for diagnosing and monitoring CKD in thin geriatric animals, especially cats and animals with other diseases that cause muscle wasting."See this Idexx article: "Introduction to a New Kidney Test: SDMA".
Polymorphisms in the canine and feline renin-angiotensin-aldosterone system genes. Kathryn M. Meurs, Lhoucine Chdid, Yamir Reina-Doreste, Joshua A. Stern. Anim. Genet. January 2015;46:224-226. Quote: We hypothesized that polymorphisms may exist in the canine and feline RAAS genes. The objective of this study was to evaluate canine and feline DNA samples for the presence of common RAAS gene polymorphisms observed in human beings. Methods: DNA samples from 15 cats of five breeds and 15 dogs of three breeds (... Cavalier King Charles Spaniel) were evaluated. Genomic DNA samples were prepared from whole blood samples. Results/conclusions: Polymorphisms were identified in the majority of animals evaluated. Most of the polymorphisms were single base pair variants although two deletions were observed in intron 16 of the feline ACE gene. Four polymorphisms changed an amino acid, but all were predicted to be benign changes based on the POLYPHEN-2 analysis. No variants were shared between species, and no previously reported human RAAS gene polymorphisms were identified. Although polymorphisms were identified, the functional importance of these changes has yet to be determined. Characterizing the significance of these polymorphisms will require additional functional and clinical studies. Nonetheless, the RAAS genes of dogs and cats are polymorphic, and further studies to evaluate the functional importance of the polymorphisms observed are warranted.
Njursjukdomar hos shetland sheepdog och cavalier king charles spaniel. Lynn Hellstrom. Swedish Univ. 2015. Quote:The kidneys are two important and multifunctional organs. All diseases that may affect the kidneys can cause a loss of kidney function. The extent of kidney function loss will have an impact on the animals' health and survival. An early recognition is therefore crucial. Inherited kidney diseases are in most cases progressive and ultimately fatal. An increased knowledge and awareness of hereditary kidney diseases might contribute to a diminished suffering and increased welfare for dogs in affected breeds, through the possibilities of performing improved breeding programs. In a (to date unpublished) Swedish study, Shetland Sheepdogs (SHS) and Cavalier King Charles Spaniel (CKCS) were identified as two of the dog breeds with the highest incidence of kidney disease in a large population of Swedish insured dogs. Neither one of these breeds are commonly mentioned as particularly affected by kidney diseases in the literature of veterinary medicine. The purpose of this study was to try to identify which kidney diseases that had been diagnosed in these two dog breeds based on the medical records from two big Swedish animal hospitals (Evidensia Sodra Djursjukhuset and Universitetsdjursjukhuset, Swedish University of Agricultural Science), and to investigate if any particular disease seemed to appear more often. The dogs included in this study were found through searches for kidney disease specific diagnostic codes in the hospital's medical record data bases. The selected medical records were carefully scrutinized and the dogs were grouped, within the breeds, for gender, age when signs first were noticed and the diagnostic code used. By studying the medical records further, in some cases, a more specific kidney disease was diagnosed, which sometimes differed considerably from the diagnostic code used. The results of the study were that no specific etiology of kidney disease was diagnosed more often than others in neither SHS nor CKCS dogs. Renal dysplasia was diagnosed in dogs of both breeds. Further studies are needed to investigate if renal dysplasia is a common problem in these breeds.
Cardiovascular-renal axis disorders in the domestic dog and cat: a veterinary consensus statement. J. L. Pouchelon, C. E. Atkins, C. Bussadori, M. A. Oyama, S. L. Vaden, J. D. Bonagura, V. Chetboul, L. D. Cowgill, J. Elliot, T. Francey, G. F. Grauer, V. Luis Fuentes, N. Sydney Moise, D. J. Polzin, A. M. Van Dongen, N. Van Israël. J.Small Animal Prac. September 2015;56(9):537-552. Quote: "Objectives: There is a growing understanding of the complexity of interplay between renal and cardiovascular systems in both health and disease. The medical profession has adopted the term "cardiorenal syndrome" (CRS) to describe the pathophysiological relationship between the kidney and heart in disease. CRS has yet to be formally defined and described by the veterinary profession and its existence and importance in dogs and cats warrant investigation. The CRS Consensus Group, comprising nine veterinary cardiologists and seven nephrologists from Europe and North America, sought to achieve consensus around the definition, pathophysiology, diagnosis and management of dogs and cats with "cardiovascular-renal disorders" (CvRD). To this end, the Delphi formal methodology for defining/building consensus and defining guidelines was utilised. Methods: Following a literature review, 13 candidate statements regarding CvRD in dogs and cats were tested for consensus, using a modified Delphi method. As a new area of interest, well-designed studies, specific to CRS/CvRD, are lacking, particularly in dogs and cats. Hence, while scientific justification of all the recommendations was sought and used when available, recommendations were largely reliant on theory, expert opinion, small clinical studies and extrapolation from data derived from other species. Results: Of the 13 statements, 11 achieved consensus and 2 did not. The modified Delphi approach worked well to achieve consensus in an objective manner and to develop initial guidelines for CvRD. Discussion: The resultant manuscript describes consensus statements for the definition, classification, diagnosis and management strategies for veterinary patients with CvRD, with an emphasis on the pathological interplay between the two organ systems. By formulating consensus statements regarding CvRD in veterinary medicine, the authors hope to stimulate interest in and advancement of the understanding and management of CvRD in dogs and cats. The use of a formalised method for consensus and guideline development should be considered for other topics in veterinary medicine. ... This consensus statement is meant to increase the awareness of and codify the definition, classification and means of identification and provide provisional information on management of CvRD."
Rubber jaw syndrome in a Cavalier King Charles Spaniel. Judith Visser. NZVA Companion Anim. Qrty. September 2015;26(3):16-20. Quote: This case describes an unusual onset of mandibular fibrous osteodystropny (i.e. 'rubber jaw') in an adult dog, as a result of renal secondary hyperparathyroidism that was induced by CKD [chronic kidney disease]. ... A 5-year-old female spayed Cavalier King Charles Spaniel was presented ... for anorexia of a 4-day period. ... The flexibility of the jaw and instability of the teeth suggested metabolic bone disease. The main differential diagnoses included primary hyperparathyroidism, secondary nutritional hyerparathyroidism and secondary renal hyperparathyroidism. ... Clinicopathologic abnormalities in serum biochemistry ... included marked azotemia, mild hypoproteinemia, mild hyperamylasemia, mildly increased total calcium, moderate hyperphosphatemia, and mild hypcholoremia. Radiographs of the skull were taken and evaluated. These showed that the bone density of the maxillae and mandibles was severely reduced. An ill-defined periosteal reaction was present along the dorsal aspect of the cranium with adjacent ill-defined cortical thinning. A dorsoventral view of the entire body was also taken. This revealed that the cortices of the radius and ulna were noticeably thinned. Due to financial constraints an ionised calcium level [and serum PTH] was not done in this dog, but the markedly elevated phosphorous level made primary hyperparathyroidism unlikely. A provisional diagnosis of end-stage renal failure with secondary renal hyperparathyroidism waas made based on the presence of marked azotemia, hyperphosphatemia and osteopenia. ... Necropsy revealed that while fractures were not present, the mandibles, maxillae and ribs were severely pliable and easy to bend. Multifocal calcium deposits were present on the parietal pleura, the kidneys were small and pale, and the parathyroid glands were severely enlarged. ... A diagnosis of chronic end-stage renal failure with severe metastatic calcification and fibrous osteodystrophy was made.
Chronobiology and Pharmacologic Modulation of the Renin-Angiotensin-Aldosterone System in Dogs: What Have We Learned? Jonathan Paul Mochel, Meindert Danhof. Reviews of Physiology, Biochemistry & Pharmacology. October 2015. Quote: "Congestive heart failure (CHF) is a primary cause of morbidity and mortality with an increasing prevalence in human and canine populations. Recognition of the role of renin-angiotensin-aldosterone system (RAAS) overactivation in the pathophysiology of CHF has led to significant medical advances. By decreasing systemic vascular resistance and angiotensin II (AII) production, angiotensin-converting enzyme (ACE) inhibitors such as benazepril improve cardiac hemodynamics and reduce mortality in human and dog CHF patients. Although several experiments have pointed out that efficacy of ACE inhibitors depends on the time of administration, little attention is paid to the optimum time of dosing of these medications. A thorough characterization of the chronobiology of the renin cascade has the potential to streamline the therapeutic management of RAAS-related diseases and to help determining the optimal time of drug administration that maximizes efficacy of ACE inhibitors, while minimizing the occurrence of adverse effects. We have developed an integrated pharmacokinetic-pharmacodynamic model that adequately captures the disposition kinetics of the paradigm drug benazeprilat, as well as the time-varying changes of systemic renin-angiotensin-aldosterone biomarkers, without and with ACE inhibition therapy. Based on these chronobiological investigations, the optimal efficacy of ACE inhibitors is expected with bedtime dosing. The data further show that benazepril influences the dynamics of the renin-angiotensin-aldosterone cascade, resulting in a profound decrease in AII and aldosterone (ALD), while increasing renin activity for about 24 h. From the results of recent investigations in human, it is hypothesized that reduction of AII and ALD is one of the drivers of increased survival and improved quality of life in dogs receiving ACE inhibitors. To support and consolidate this hypothesis, additional efforts should be directed toward the collection of circulating RAAS peptides in spontaneous cases of canine CHF. If such a link could be established, profiling of these biomarkers could support determination of the severity of heart failure, complement clinical and echocardiographic findings, and be used for therapeutic drug monitoring purposes. ... The research summarized herein is not a static and completed piece of work but is, instead, a starting point for further data integration and hypothesis testing."
Effects of high doses of enalapril and benazepril on the pharmacologically activated renin-angiotensin-aldosterone system in clinically normal dogs. Marisa K. Ames, Clarke E. Atkins, Seunggon Lee,Andrea C. Lantis, James R. zumBrunnen. Am. J. Vet. Res. December 2015;76(12):1041-1050. Quote: "Objective: To determine whether high doses of enalapril and benazepril would be more effective than standard doses of these drugs in suppressing the furosemide-activated renin-angiotensin-aldosterone system (RAAS). Animals: 6 healthy Beagles. Procedures: 2 experiments were conducted; each lasted 10 days, separated by a 2-week washout period. In experiment 1, all dogs received furosemide (2 mg/kg, PO, q 12 h) and enalapril (1 mg/kg, PO, q 12 h) for 8 days (days 0 through 7). In experiment 2, dogs received furosemide (2 mg/kg, PO, q 12 h) and benazepril (1 mg/kg, PO, q 12 h) for 8 days. Effects on the RAAS were determined by assessing serum angiotensin-converting enzyme (ACE) activity on days −1, 3, and 7; serum aldosterone concentration on days −2, −1, 1, 3, and 7; and the urinary aldosterone-creatinine ratio (UAldo:C) in urine collected in the morning and evening of days −2, −1, 1, 3, and 7. Results: High doses of enalapril and benazepril caused significant reductions in serum ACE activity on all days but were not more effective than standard doses used in other studies. Mean UAldo:C remained significantly higher on days 2 through 7, compared with baseline values. Serum aldosterone concentration also increased after drug administration, which mirrored changes in the UAldo:C. Conclusions & Clinical Relevance: In this study, administration of high doses of enalapril and benazepril significantly inhibited ACE activity, yet did not prevent increases in mean urine and serum aldosterone concentrations resulting from furosemide activation of RAAS. This suggested that aldosterone breakthrough from ACE inhibition was a dose-independent effect of ACE inhibitors."
Early Diagnosis of Chronic Kidney Disease in Dogs & Cats: Use of Serum
Creatinine & Symmetric Dimethylarginine. Gregory F. Grauer. Today's
Vet. Pract. March 2016;6(2). Quote: Incorporating evaluation of serum SDMA
[serum symmetric dimethylarginine] along with closer monitoring of sCr may
facilitate early diagnosis of CKD in dogs and cats. In general, diagnostic
results that indicate increased monitoring include:
• sCr concentrations
of 1.4 mg/dL or greater in dogs or 1.6 mg/dL or greater in cats
• Serum
SDMA concentrations greater than 14 mcg/dL.
Longitudinal assessment of
these measures almost always provides better data than one-time evaluations.
No single laboratory test is perfect; trending laboratory data, with the
same test method, tends to improve diagnostic sensitivity.
Prevalence of pancreatic, hepatic and renal microscopic lesions in post-mortem samples from Cavalier King Charles Spaniels. Kent, Andrew C. C.; Constantino-Casas, Fernando; Rusbridge, Clare; Corcoran, Brendan; Carter, Margaret; Ledger, Tania; Watson, Penny J. J. Small Animal Practice. April 2016;57(4):188-193. Quote: "Objectives: To describe the prevalence of pancreatic, hepatic and renal microscopic lesions in post-mortem samples from [54] Cavalier King Charles Spaniels (CKCS) presented to a UK post-mortem collection scheme [The Cavalier Collection Scheme]. Methods: Histopathology was performed on the organs of interest and the prevalence of microscopic lesions described, this was related back to the clinical signs shown ante-mortem. Results: Evidence of chronic pancreatitis was seen in 51.9% of the cases, and age correlated with severity of disease, suggesting that chronic pancreatitis is a progressive condition. Evidence of renal lesions was present in 52.2% of cases, most commonly inflammatory disease. The rate of ante-mortem diagnosis was low for both pancreatic and renal disease, at 25% and 16.7% respectively. Primary hepatic lesions were present in only 11.1% of cases, but secondary hepatic lesions were more common and were present in 64.8%. ... This is the first study to report the prevalence of renal disease in CKCS. Glomerulonephritis or interstitial nephritis was identified in 19 dogs (41%) in this study, whilst only four dogs had an antemortem diagnosis of renal disease. Twenty-four dogs (44·4%) in this study had congestive heart failure and were on long-term medication for cardiac disease, and this may mean that subtle changes in renal function were overlooked. Many of the dogs were on medications that may have had an impact on renal perfusion, for example, angiotensin converting enzyme inhibitors (ACEi) and diuretics, and the assumption may have been that mild azotaemia resulted from reduced renal perfusion or the effects of diuresis. However, the changes identified on histopathology were indicative of primary renal disease in many dogs. It is likely that at least some dogs with glomerulonephritis had measurable proteinuria, and some dogs with nephritis had poorly concentrated urine. ... Clinical Significance: Pancreatic and renal lesions are common in Cavalier King Charles Spaniels and clinicians should be aware of this when presented with clinical cases, they have similar rates of hepatic disease as the general population."
Advances in the evaluation of canine renal disease. Rachel Cianciolo, Jessica Hokamp, Mary Nabity. Vet. J. September 2016;215(Special Issue):21-29. Quote: "Many recent advances in the evaluation of dogs with kidney disease have improved our diagnostic algorithms and impacted our therapeutic strategies. Non-invasive techniques, such as urinary and serologic biomarker evaluation, can help a clinician diagnose and treat a patient that cannot undergo a renal biopsy for clinical or financial reasons. Some biomarkers might help localize the affected structure (glomerulus vs. tubule) and indicate the type or severity of injury present. Although more research is needed, studies indicate that some biomarkers (e.g. urine protein to creatinine ratio and urinary immunoglobulins) can be useful in predicting adverse outcomes. Importantly, the sensitivity and specificity of biomarkers for renal injury should be established and clinicians need to understand the limitations of these assays. If a renal biopsy is performed, then it should be evaluated by a specialty diagnostic service with expertise in nephropathology. A panel of special stains, immunofluorescence for the detection of immunoglobulins and complement factors, and transmission electron microscopy can be routinely employed in cases of glomerular disease. These advanced diagnostics can be used to detect immune deposits in order to definitively diagnose immune complex mediated glomerular disease. Integrating the results of biomarker assays and comprehensive renal biopsy evaluation, the clinician can make informed therapeutic decisions, such as whether or not to immunosuppress a patient. Highlights: • Urinary biomarker analysis is a non-invasive way to help determine the type and severity of renal injury. • Serum biomarkers are used to detect decreased glomerular filtration rate. • Comprehensive evaluation of renal tissue definitively diagnoses immune complex mediated glomerular disease."
Evaluation of serum cystatin-C and symmetric dimethylarginine concentrations in dogs with heart failure from chronic mitral valvular insufficiency. Bum-Sul Choi, Hyeong-Sun Moon, Sang-Hyuk Seo, Changbaig Hyun. Vet. Med. Sci. September 2016. Quote: Reduction in glomerular filtration rate (GFR) is a common complication in advanced stages of heart failure (HF). The convenient and precise assessment for GFR would be useful for early detection of renal impairment in HF dogs. Our hypothesis of this study was the GFR would be reduced in advanced stages of HF from chronic mitral valvular disease (CMVI), as indicated by renal markers including serum cystatin-C (Cys-C) and symmetric dimethylarginine (SDMA) concentrations. Forty-three client-owned dogs consisting of 33 dogs with different stages of HF from CMVI and 10 age-matched healthy dogs were enrolled in this study. Serum Cys-C and SDMA concentrations along with other renal (i.e., urea nitrogen and creatinine) and echocardiographic markers were evaluated in healthy and CMVI dogs. Serum Cys-C concentrations were 1.4 ± 0.4 mg/l in control, 2.1 ± 0.9 mg/l in ISACHC I, 2.9 ± 0.8 mg/l in ISACHC II and 3.6 ± 0.6 mg/l in ISACHC III dogs, whereas serum SDMA concentrations were 8 ± 2 ug/dl in control, 14 ± 3 ug/dl in ISACHC I, 18 ± 6 ug/dl in ISACHC II and 22 ± 7 ug/dl in ISACHC III dogs. There was close correlation of serum Cys-C and SDMA concentrations to serum creatinine, urea nitrogen and the severity of HF. Our study demonstrated that the GFR was decreased in dogs with CMVI having earlier stages of HF.
Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Roberta Relford, Jane Robertson, Celeste Clements. Small Animal Pract. November 2016;46(6):941-960. Quote: Symmetric dimethylarginine (SDMA) is a new kidney biomarker that accurately reflects glomerular filtration fate (GFR). SDMA level increases earlier in chronic kidney disease (CKD), on average with 40% reduction of GFR, compared with up to 75% reduction needed to increase creatinine level. Unlike creatinine, SDMA is not affected by lean body mass so it is a more sensitive indicator of kidney function in patients with muscle loss. ... In a group of Cavalier King Charles spaniels, SDMA level was not affected by age or asymptomatic mitral regurgitation. [citing this 2006 article above] ... The validated immunoassay for SDMA, the IDEXX SDMA test, is a clinically relevant and reliable tool for diagnosing early CKD in small animals when creatinine level is still within the reference interval. SDMA was added to the International Renal Interest Society CKD guidelines to complement creatinine testing in staging early and advanced disease.
Cardiorenal Syndrome: Diagnosis and Management. João S. Orvalho, Larry D. Cowgill. Vet. Clinics of No. Amer.: Sm. Anim. Pract. September 2017;47(5):1083-1102. Quote: Introduction: The definition of cardiorenal syndrome (CRS) includes a variety of acute or chronic conditions, in which the primary failing organ can be the heart, the kidney, or both (due to an independent systemic condition), and how dysfunction of one organ system affects injury to or function of the other organ system. The Cardiovascular-Renal Axis Disorders (CvRDs) Consensus Group proposed that CvRDs are defined as disease-induced, toxin-induced, or drug-induced structural and/or functional damage to the kidney or to the cardiovascular system leading to disruption of the normal interactions between these systems, to the ongoing detriment of one or both. Conclusion: An accurate appreciation of the kidney and the cardiovascular system and their interactions may have practical clinical implications. A multidisciplinary evaluation, including the expertise of cardiologists and nephrologists, may be the most appropriate approach for patients with concurrent cardiac and kidney disease or predisposed to CRS. The outcome of patients with CRS is likely to improve with the increasing awareness and ability to identify and understand the pathophysiological characteristics of CRS. The greater utilization of existing and emerging organspecific biomarkers with greater sensitivities than conventional diagnostics forecast new opportunities to diagnose and manage cardiac disease.
Angiotensin-converting enzyme activity and inhibition in dogs with cardiac disease and an angiotensin-converting enzyme polymorphism. Kathryn M Meurs, Joshua A Stern, Clarke E Atkins, Darcy Adin, Brent Aona, Julia Condit, Teresa DeFrancesco, Yamir Reina-Doreste, Bruce W Keene, Sandy Tou, Jessica Ward, Kathleen Woodruff. J. Renin-Angiotensin-Aldosterone Sys. October 2017:18(4)1-4. Quote: Objective: The objective of this study was to evaluate angiotensin-converting enzyme (ACE) activity in dogs and with and without an ACE polymorphism in the canine ACE gene, before and after treatment with an ACE inhibitor. ... In humans, an ACE gene insertion/deletion (I/D) in intron 16, has been shown to impact ACE activity with the deletion allele (D) associated with higher levels of ACE activity then the insertion allele (I). The polymorphism has been associated with a variable response to ACE inhibitors. Humans with this polymorphism have increased baseline ACE activity, increased levels of angiotensin II production and require higher doses of ACE inhibitors to achieve an adequate response to ACE inhibition. We have previously identified a single nucleotide polymorphism (SNP) in the same intron in the canine ACE gene. We hypothesized that this SNP in the canine ACE gene could be associated with a variable response to ACE inhibitor therapy in the dog. ... In the study presented here we evaluated dogs with myxomatous mitral valve disease, the most common form of heart disease in the dog, with and without the ACE gene polymorphism and assessed ACE activity and systolic blood pressure before and after ACE inhibition with enalapril. ... Methods: Thirty-one dogs [10 cavalier King Charles spaniels] (20 wild-type [4 CKCSs], 11 ACE polymorphism [6 CKCSs]) with heart disease were evaluated with ACE activity measurement and systolic blood pressure before and after administration of an ACE inhibitor (enalapril). ... There was no significant difference in cardiac enlargement between the two groups. The median VHS for the wild-type dogs was 11.3 (11.2-12.2), and the median VHS for the ACE polymorphism dogs was 11.8 (11.29-13.04). Results: Median pre-treatment ACE activity was significantly lower for ACE polymorphism dogs than for dogs with the wild-type sequence. After two weeks of an ACE inhibitor, ACE activity was significantly reduced for both genotypes; mean post-therapy ACE activity was no different between the groups. Conclusion: An ACE polymorphism is associated with lower levels of ACE activity. Dogs with the polymorphism still experience suppression of ACE activity in response to an ACE inhibitor. It is possible that the genetic status and ACE activity of dogs may impact the response of dogs with this variant to an ACE inhibitor. ... In this study, 12 different breeds of dogs were evaluated. The ACE polymorphism was identified in five of the 12 breeds, but was observed most commonly in the Cavalier King Charles spaniel (six of 10 dogs).
Measurement of urea and creatinine in saliva of dogs: a pilot study. Asta Tvarijonaviciute, Luis Pardo-Marin, Fernando Tecles, Juana Dolores Carrillo, Juan Diego Garcia-Martinez, Luis Bernal, Josep Pastor, Jose J. Cerón, Silvia Martinez-Subiela. BMC Vet. Res. July 2018;14:223. Quote: Background: Urea and creatinine in saliva have been reported to be possible markers of chronic kidney disease (CKD) in humans. The aim of this study was to assess if urea and creatinine could be measured in canine saliva, and to evaluate their possible changes in situations of CKD. Results: The spectrophotometric assays for urea and creatinine measurements in saliva of dogs showed intra- and inter-assay imprecision lower than 12% and coefficients of correlation close to 1 in linearity under dilution tests. Healthy dogs showed median salivary concentrations of urea of 39.6 mg/dL and creatinine of 0.30 mg/dL, whereas dogs with CKD showed median salivary urea of 270.1 mg/dL and creatinine of 1.86 mg/dL. Positive high correlations were found between saliva and serum activities of the two analytes (urea, r = 0.909; P < 0.001; creatinine, r = 0.819; P < 0.001). Conclusions: Urea and creatinine concentrations can be measured in canine saliva with commercially available spectrophotometric assays. Both analytes showed higher values in saliva of dogs with CKD compared with healthy dogs and their values were highly correlated with those in serum in our study conditions.
Reliability of symmetric dimethylarginine in dogs with myxomatous mitral valve disease as kidney biomarker. Alice Savarese, Monica Probo, Chiara Locatelli, Sergio Aurelio Zanzani, Alessia Libera Gazzonis, Melissa Papa, Paola Giuseppina Brambilla. Open Vet. J. August 2018;8(3):318-324. Quote: The most common cause of heart failure in the canine population is myxomatous mitral valve disease, sometimes complicated by chronic kidney disease. Many studies have been done on the use of symmetric dimethylarginine as biomarker of renal impairment in dogs affected by chronic kidney disease, few studies have examined his reliability as biomarker in dogs affected by heart diseases. Aim of this study was to evaluate symmetric dimethylarginine in dogs affected by mitral valve disease in order to assess his reliability in heart diseases. This was a retrospective case-control study on a clinical population of dogs affected by mitral valve disease (cases) vs healthy dogs (controls). Both groups underwent a complete physical evaluation, echocardiographic examination, complete blood count, biochemical panel, including serum creatinine and urea and urine analysis with protein-to-creatinine ratio. Serum was frozen and sent to IDEXX laboratories for symmetric dimethylarginine determination. General linear model was applied to data. A total number of 24 cases [including two cavalier King Charles spaniels -- 8%] and 7 controls were included. Symmetric dimethylarginine value was in the reference value in the 75% (n=18) of cases, and in the 43% (n=3) of controls. Once set symmetric dimethylarginine as dependent variable, no statistical significant differences were found for each variable considered (breed, age, sex, weight, class of cardiomyopathy, presence/absence of valvular disease, presence/absence of congestive heart failure, pharmacological therapy, creatinine and urea concentration). Blood concentration of SDMA resulted not influenced by the variables mentioned above, so it could be considered a reliable marker of early renal impairment in dogs affected by mitral valve disease. ... In conclusion, our study showed that SDMA is free from correlation with breed, age, sex, weight, presence/absence of MMVD, presence of CHF symptoms and pharmacological therapy as well. SDMA can be actually considered a reliable parameter for evaluation of renal function in dogs affected by MMVD, especially in those patients with a non-advanced stage of disease (ACVIM class B2), for which an early diagnosis of the onset of kidney failure is fundamental in order to plan a diuretic therapy. SDMA repeated measurements over time, as recommended by IRIS guidelines, are necessary (IRIS, 2016), because one determination does not allow us to exclude definitely a later onset of the renal impairment and then to be considered diagnostic in order to highlight a possible onset of CRS.
The renin-angiotensin-aldosterone system and its suppression. Marisa K. Ames, Clarke E. Atkins, Bertram Pitt. J. Vet. Intern. Med. February 2019; doi: 10.1111/jvim.15454 Quote: Chronic activation of the renin-angiotensin-aldosterone system (RAAS) promotes and perpetuates the syndromes of congestive heart failure, systemic hypertension, and chronic kidney disease. Excessive circulating and tissue angiotensin II (AngII) and aldosterone levels lead to a pro-fibrotic, -inflammatory, and -hypertrophic milieu that causes remodeling and dysfunction in cardiovascular and renal tissues. Understanding of the role of the RAAS's in this abnormal pathologic remodeling has grown over the past few decades and numerous medical therapies aimed at suppressing the RAAS have been developed. Despite this, morbidity from these diseases remains high. Continued investigation into the complexities of the RAAS should help clinicians modulate (suppress or enhance) components of this system and improve quality of life and survival. This review focuses on updates in our understanding of the RAAS and the pathophysiology of AngII and aldosterone excess, reviewing what is known about its suppression in cardiovascular and renal diseases, especially in the cat and dog. ... Recently, a single nucleotide polymorphism in intron 16 of the ACE gene in a group of 31 dogs (including 10 CKCS) has been described. (See this October 2017 article.) The ACE activity in dogs with the polymorphism was significantly lower than those without, yet both groups had significant suppression of ACE activity after 2 weeks of ACEI treatment. Six of the 10 CKCS carried the polymorphism, which provides support to the concept, held by some, that the RAAS of the CKCS may not truly represent the species as a whole. Further study is necessary to determine whether differences in RAAS phenotypes impact the response to RAAS suppressive treatment and the natural history of cardiac disease in this breed.
Symmetric dimethylarginine in dogs with myxomatous mitral valve disease at various stages of disease severity. Carlotta Valente, Carlo Guglielmini, Oriol Domenech, Barbara Contiero, Eric Zini, Helen Poser. PLoS ONE. September 2020;15(9): e0238440. Quote: Symmetric dimethylarginine (SDMA) is a serum biomarker of renal damage in dogs. Moreover, SDMA concentration is an independent predictor of development of severe heart failure (HF) in humans with cardiac disease. This study evaluates whether the serum concentration of SDMA in dogs with myxomatous mitral valve disease (MMVD) is influenced by the severity of heart disease, pulmonary hypertension (PH) and treatment of HF. A total of 99 client-owned dogs [including 3 cavalier King Charles spaniels, 50 mongrels, and 46 of 25 other breeds] were included in this retrospective case-control study; 78 dogs were affected by MMVD [incuding the 3 CKCSs] and classified according to the American College of Veterinary Internal Medicine (ACVIM) guidelines, and 21 were healthy controls. ... For each dog, history, physical examination, complete blood count, biochemical profile, thoracic radiography, 6-lead standard electrocardiogram and trans-thoracic echocardiography were available. Comparisons were performed between groups of dogs belonging to different ACVIM stages and between dogs with and without PH. ... The severity of the MMVD was assessed according to ACVIM guidelines. In particular, asymptomatic dogs without radiographic or echocardiographic evidence of cardiac remodeling were considered to be in stage B1; asymptomatic dogs with signs of cardiac remodeling [i.e., cardiomegaly with left atrium (LA) and/or left ventricle (LV) enlargement] were considered to be in stage B2; symptomatic dogs in which at least one episode of pulmonary edema and/or pleural effusion due to HF had occurred were considered to be in stage C, while symptomatic dogs with end-stage disease and refractory to standard therapies were considered to be in stage D. ... Left ventricular enlargement was considered for values of N-LVIDd > 1.7. The left atrium (LA) to aorta (Ao) ratio was determined using the right parasternal short axis view at the heart base level as previously described and LA enlargement was noted when LA/Ao > 1.6. ... The results of this study failed to demonstrate a significant increase in SDMA concentration with the progression of the disease stage in dogs with MMVD. ... The median SDMA concentration was neither significantly different among groups of dogs in different disease stages, nor among dogs with MMVD, nor between those with [14.5 μg/dl (10.5-18.8)] and without PH [13 μg/dl (9-17.2)]. The concentration of SDMA did not differ between dogs when considering the combined effect of the ACVIM group and cardiac treatment. Furthermore, no correlation was found between SDMA concentration and radiographic and echocardiographic parameters associated with increased MMVD severity. In conclusion, the results of this study failed to demonstrate that renal function, evaluated by measuring serum SDMA concentration, is significantly impaired in dogs with MMVD. Although some dogs in the ACVIM Stages] C+D group of MMVD had an increased concentration of the variables used to identify renal dysfunction, this was most likely due to pre-renal azotemia instead of representing a feature of the CRS [cardiorenal syndrome] described in humans.
The new age of renal biomarkers: does SDMA solve all of our problems? H. J. Sargent, J. Elliott, R. E. Jepson. J. Sm. Anim. Pract. November 2020; doi: 10.1111/jsap.13236. Quote: Within clinical small animal practice, diagnosis of both chronic kidney disease and acute kidney injury is common. To assess renal function, measurement of glomerular filtration rate is considered the gold standard. Currently, routine tests of kidney function include surrogate markers of glomerular filtration rate such as serum creatinine, and urea, each with their own limitations, whilst urine protein to creatinine ratio gives an indication of glomerular and tubular handling of protein, and urine specific gravity information about urine concentrating ability by the kidney. These parameters are used together with historical and physical examination data to give a diagnosis of kidney disease following which creatinine, proteinuria and blood pressure are used to stage chronic kidney disease and, together with urine output, grade acute kidney injury according to the International Renal Interest Society. However, there has been much concern that creatinine is insensitive when used to indicate early decline in renal function and this has highlighted the need for additional methods of diagnosing and monitoring these patients, with the potential to allow earlier therapeutic intervention. Symmetric dimethylarginine is a novel biomarker, which has been shown to perform as a surrogate marker of glomerular filtration rate in small animals. This article will review current research on symmetric dimethylarginine and the ways in which it may be utilised in small animal practice; current research supports the use of symmetric dimethylarginine as a screening test for detection of early chronic kidney disease according to International Renal Interest Society guidelines, but further research is required in to the usefulness of symmetric dimethylarginine as a tool for monitoring disease and the effect of non‐renal influences.
Contribution of Chronic Kidney Disease to the Progression of Myxomatous Mitral Valve Disease in Dogs. Jeong-Sook Oh, Dohee Lee, Taesik Yun, Yoonhoi Koo, Yeon Chae, Dongwoo Chang, Byeong-Teck Kang, Mhan-Pyo Yang, Hakhyun Kim. 2021 ACVIM Forum. June 2021; C23. Quote: In veterinary medicine, the understanding of the interation between renal and cardiovascular system is increasing, but it has not yet been completely elucidated. Therefore, in this study, we aimed to investigate the cardiorenal system in dogs by evaluating chronic kidney disease (CKD) as a risk factor for the progression of myxomatous mitral valve disease (MMVD). The medical records of 63 dogs diagnosed with MMVD stage B1, B2, or C, according to the guidelines of the American College of Veterinary Internal Medicine, were retrospectively reviewed. The progression of MMVD was examined 6 months after the first diagnosis, and the mortality rate was recorded. The indicators for the progression of MMVD and mortality rate were compared between the dogs with only MMVD (MMVD group) and the dogs with both CKD and MMVD (concurrent group). In MMVD stages B2 and C, change in vertebral heart score was significantly greater in the concurrent group than in the MMVD group. In all stages of MMVD, the concurrent group showed a greater change in left ventricular end-diastolic diameter normalized for body weight than the MMVD group. However, no significant differences were found in the progression of murmur grade and left atrium/aorta ratio between the two groups. The mortality of the concurrent group was significantly higher than that of the MMVD group. These results provide an insight into the relationship between CKD and MMVD, and suggest the potential role of CKD as a risk factor for the progression of canine MMVD.
Is There an Association Between Breed, Age, and Sex with High and Low Serum Creatinine Levels in Dogs? - From the Analysis of Electronic Medical Record Data. Akiko Uemura, Lina Hamabe, Ryou Tanaka, Noriko Tanaka, Tsuyoshi Takizawa, Toshiro Iwasaki. Kafkas Univ. Vet. Fak. Derg., January 2022; doi: 10.9775/kvfd.2021.26490. Quote: Medical record data were analyzed for serum creatinine level in dogs to determine useful associations between the data. Differences in sex, age, and breed were analyzed using Fisher's exact test, and multiple factors such as sex were analyzed by contingency table analysis. Of the 3347 dogs [located in Japan between June 2008 and November 2016] that were tested for serum creatinine level, 243 dogs [7.3%] had creatinine over 1.4 mg/dL more than once. The overall rate of renal dysfunction in all breeds was 7.3%, but the rate for cavalier King Charles Spaniels was 14.1%, and for Shetland Sheepdogs it was also 14.1%, both significantly higher than the overall rate. ... Table 3. ... Cavalier King Charles Spaniel: Study Group (a) 64; High CRE Group (b) 16; (b/a) 14.1%. ... The high rate of high CRE seen in Cavalier King Charles Spaniels has also been reported in a British study. This may be connected to the fact that this breed is at high risk of mitral insufficiency.
Long-term outcome of dogs recovering from acute kidney injury: 132 cases. Mali Bar-Nathan, Hilla Chen, Dar Rimer, Gilad Segev. J. Vet. Intern. Med. April 2022; doi: 10.1111/jvim.16435. Quote: Background: Information regarding long-term outcome of dogs recovering from acute kidney injury (AKI) is limited. Objectives: Determine the long-term outcome of dogs recovering from AKI and identify predictors for serum creatinine concentration (sCr) normalization and long-term outcome. Animals: One hundred thirty-two dogs with AKI that survived >30 days postdischarge. ... The most common breeds were mixed (61 dogs; 46%), German shepherd (9 dogs, 7%), Cavalier King Charles spaniel and Yorkshire terrier (5 dogs each, 4%) ... Methods: Retrospective study. Search of medical records of dogs diagnosed with AKI that survived to discharge. Follow-up data were retrieved from medical records and by telephone interviews with the owners or primary care veterinarians or both. Results: Estimated median survival time (MST) was 1322 days (95% confidence interval [CI], 1147-1626), and 76% of the dogs were alive at last contact. Normalization of sCr was documented in 55% of the dogs at discharge and in additional 20% during the follow-up period. The proportion of dogs with sCr normalization decreased with increase in AKI grade. Long-term survival was not associated with sCr normalization. Etiology was associated with the long-term outcome. Conclusion and Clinical Importance: Long-term survival of dogs with AKI is longer than previously described. Normalization of sCr in 99 dogs (75%) occurred, either at discharge or within the follow-up period. Normalization of sCr was not associated with long-term survival. Estimated MST of dogs with sCr normalization was not different compared with dogs that developed azotemic chronic kidney disease (CKD), presumably because of slow CKD progression rate. Etiology is an important factor determining sCr normalization and long-term survival, emphasizing the importance of the reversibility of renal injury rather than its severity.
Congenital bilateral renal dysplasia in a Cavalier King Charles Spaniel. Nicole V. Tusa, Nyssa A. Levy. Vet. Rec. May 2022; doi: 10.1002/vrc2.390. Quote: This report describes a case of congenital bilateral renal dysplasia in a Cavalier King Charles Spaniel (CKCS) puppy, which has not been previously reported in the breed. An 8-week-old male intact CKCS was presented to a University Teaching Hospital for the evaluation of vomiting. He was obtained from a pet store approximately 3 days prior to presentation, and his owners reported that he had foul smelling breath and was unwilling to eat, yet was playful. He started vomiting 1 day prior to presentation. His breeding information was unavailable but he had received vaccinations for distemper and parvovirus, as appropriate for his age. No leptospirosis vaccinations were given. His past medical history included a surgically corrected umbilical hernia and intestinal parasitism treated with dewormer. There was no known toxin exposure. Lab work revealed azotemia [, hyperphosphatemia and panhypoproteinemia]. The puppy was treated with medical management involving fluid therapy, antibiotics, and supportive care. Due to the development of unresponsive oliguric renal failure, his level of care intensified and peritoneal dialysis was initiated and continued over a 12-day course. After initiating peritoneal dialysis, azotemia improved but the patient acutely declined after an episode of regurgitation and ultimately cardiopulmonary arrest. Post-mortem examination revealed bilateral persistent fetal glomeruli, tubular dysplasia, and rare tubular necrosis. While congenital renal dysplasia has been reported in other canine breeds, to the authors' knowledge, this is the first report of congenital renal dysplasia in a CKCS. ... Congenital bilateral renal dysplasia should be considered as a differential for azotemia in Cavalier King Charles Spaniel breed. It is hoped that this report will provide an example, as well as raise awareness, for future Cavalier King Charles Spaniel puppies presenting with similar clinical signs and diagnostic findings.
Dose-response of benazepril on biomarkers of the classical and alternative pathways of the reninangiotensin-aldosterone system in dogs. Samantha Sotillo, Jessica L. Ward, Emilie Guillot, Oliver Domenig, Lingnan Yuan, Joseph S. Smith, Vojtech Gabriel, Chelsea A. Iennarella-Servantez, Jonathan P. Mochel. Res. Square. July 2022; doi: 10.21203/rs.3.rs-1858200/v1. Quote: Angiotensin-converting enzyme inhibitors (ACEI) such as benazepril are commonly prescribed in both humans and dogs with heart disease to mitigate the renin-angiotensin-aldosterone system (RAAS); however, the dose-dependent effects of benazepril on comprehensive RAAS components remain unknown. In this study, nine purpose-bred healthy dogs received three different dosages of oral benazepril (0.125 mg/kg, 0.25 mg/kg, or 0.5 mg/kg) in a randomized crossover design following induction of RAAS activation by consuming a low-sodium diet ... (Hill's Prescription Diet h/d; 17 mg sodium per 100kcal, 0.08% sodium on a dry matter basis) at 23:00 PM once daily for five days to attain a steady activation of RAAS. ... Blood samples were collected at serial time intervals after benazepril dosing to measure plasma benazeprilat (active metabolite of benazepril) and serum RAAS biomarkers. Blood pressure and echocardiogram were performed at baseline and after each benazepril administration. Time-weighted averages for RAAS biomarkers for 12 hours post-dose and hemodynamic variables were compared between dosing groups using Wilcoxon rank-sum testing. Compared to the lowest dosage of benazepril (0.125 mg/kg), the highest dosage (0.5 mg/kg) resulted in lower time-weighted average values of angiotensin (Ang) II (-38%, P = 0.004), Ang1-5 (-53%, P = 0.001), ACE-S (surrogate for ACE activity; -59%, P = 0.0002), and ALT-S (surrogate for alternative RAAS activity; -22%, P = 0.004), and higher values of AngI (+ 78%, P = 0.014) and PRA-S (surrogate for plasma renin activity; +58%, P = 0.040). There were no relevant differences between dosing groups for blood pressure or echocardiographic variables. Knowledge of dose-dependent alterations in biomarkers of the classical and alternative RAAS pathways could help inform clinical trials for dosage optimization in both dogs and humans.
Prospective evaluation of novel biomarkers of acute kidney injury in dogs following cardiac surgery under cardiopulmonary bypass. Daria Starybrat, Rosanne Jepson, Poppy Bristow, Sarah Peterson, Maha Yerramilli, Murthy Yerramilli, Yu-Mei Chang, Stefano Cortellini. Vet. Emerg. Crit. Care. September 2022; doi: 10.1111/vec.13250. Quote: Objective: To assess the occurrence of acute kidney injury (AKI) in dogs undergoing cardiac surgery under cardiopulmonary bypass (CPB) and explore associations between traditional and novel serum and urinary biomarkers. Design: Prospective cohort study conducted between July 2018 and April 2019. Setting: University teaching hospital. Animals: Nineteen dogs [including 7 cavalier King Charles spaniels (37%)] undergoing cardiac surgery under CPB with preoperative serum creatinine <140 μmol/L (<1.6 mg/dl). Interventions: Blood and urine samples were obtained at 4 time points: preoperatively following general anesthesia induction, immediately postoperatively, and 2 and 4 days postoperatively (T1, T2, T3, and T4). AKI was defined as an increase in serum creatinine >26.4 μmol/L (>0.3 mg/dl) above baseline within 48 hours. Serum creatinine, C-reactive protein (CRP), symmetric dimethylarginine (SDMA), inosine, beta-aminoisobutyric acid (BAIB), urinary clusterin (uClus), and urinary cystatin B (uCysB) were measured. Data were log-transformed (log10) when appropriate and assessed using linear mixed-effects models. Measurements and Main Results: AKI occurred in 3 of 19 dogs (15.8%, 95% confidence interval: 0.047-0.384). Inosine increased at T2 (adjusted mean ± standard error: 53 ± 5.6) in all dogs, and then gradually decreased. Log10uCysB increased at T2 (2.3 ± 0.1) in all dogs and remained high. Log10CRP and log10uClus increased significantly at T3 (1.9 ± 0.1 and 3.6 ± 0.1, respectively) in all dogs and remained increased. There was a significant positive association between serum creatinine and SDMA (estimate ± standard error: 0.06 ± 0.00), between log10CRP and log10uClus (0.35 ± 0.08), between SDMA and creatinine as well as between SDMA and BAIB (11.1 ± 0.83 and 1.06 ± 0.22, respectively) for all dogs at all time points. Conclusions: Inosine and uCysB concentrations changed in all dogs immediately following a surgery under CPB and may indicate tubular injury. Further studies are required to ascertain the usefulness of those biomarkers in early detection of AKI.
Outcome of Acquired Fanconi Syndrome Associated with Ingestion of Jerky Treats in 30 Dogs. Stinna Nybroe, Charlotte R. Bjørnvad, Camilla F. H. Hansen, Tenna S. L. Andersen, Ida N. Kieler. Animals. November 2022; doi: 10.3390/ani12223192. Quote: Acquired canine proximal renal tubulopathy (Fanconi syndrome) related to excessive ingestion of jerky treats has been recognized since 2007. This study aimed to improve knowledge about the syndrome's characteristics, especially long-term outcome. By reaching out to veterinarians and dog owners, dogs suspected of jerky induced Fanconi syndrome were identified. The dog's medical records were reviewed, and owners interviewed. Data was analyzed using linear mixed models (p < 0.05 was considered statistically significant) and descriptive statistics are reported. Thirty dogs [including 4 cavalier King Charles spaniels (13.3%)], median body weight 6.8 (range 1.2-59) kg and age 6.5 (0.5-14) years, were enrolled as suspected cases based on history of jerkey ingestion and confirmed normoglycemic/ hypoglycemic glycosuria. Clinical signs included polydipsia (23/30), polyuria (21/30), lethargy (19/30), weight loss (15/30), hyporexia (11/30), vomiting (7/30), diarrhea (7/30) and no clinical signs (2/30). Para-clinical findings included azotemia (6/28), hypophosphatemia (9/25), metabolic acidosis (3/8), hypokalemia (6/20), proteinuria (13/26), aminoaciduria (4/4), hematuria (22/29) and ketonuria (7/27). Clinical signs resolved in 22/28 within 11 (0.3-52) weeks and glycosuria resolved in 28/30 within 6.5 (1-31) weeks. There were no associations between serum creatinine and urea and the amount/duration of jerky ingestion. Serum symmetric dimethylarginine concentrations were only available for a few dogs, therefore no conclusion was achieved on a possible association with duration of jerky ingestion. Apart from a larger percentage of dogs achieving complete recovery, the current findings are in agreement with previous reports.
Cystatin C for managing diuretic-induced kidney dysfunction in MMVD dogs. Donghyun Han, Jae Hyeon Cho, Chung Hui Kim. Korean J. Vet. Service. September 2023; doi: 10.7853/kjvs.2023.46.3.181. Quote: Cystatin C, a low-molecular-weight protein synthesized by cells, is being explored as a valuable biomarker for assessing renal function in veterinary medicine. Although the relationship between cystatin C and heart disease remains unclear, some studies suggest a possible association. This retrospective case-control study aimed to investigate the role of cystatin C as a biomarker for heart disease and its correlation with diuretic use in veterinary clinical practice. A total of 39 dogs were included in this study, comprising 9 control dogs without a predisposition to heart disease and 30 dogs in the study group diagnosed with heart disease. Among the 30 dogs with heart disease, 18 exhibited symptoms indicative of heart failure. Results showed significantly higher cystatin C levels in the heart disease group compared to the control group (P<0.05). However, no significant differences were observed among different stages of heart disease severity in the control group. Furthermore, cystatin-C showed statistically positive correlations with BUN (r =0.478, P<0.01), creatinine (r =0.506, P<0.01), and furosemide (r =0.338, P<0.05). Diuretics are essential for managing congestive heart failure, and the observed associations between cystatin C and furosemide suggest potential impacts of diuretic use on renal function in dogs with heart failure. Monitoring renal function markers, such as cystatin C, can aid in predicting and managing potential renal complications, ultimately improving the overall health and quality of life of dogs with heart disease.
Factors associated with thrombotic disease in dogs with renal proteinuria: A retrospective of 150 cases. Luca Fortuna, Harriet M. Syme. J. Vet. Intern. Med. December 2023; doi: 10.1111/jvim.16973. Quote: Background: Knowledge of additional risk factors for thrombotic disease (TD) among dogs with renal proteinuria is limited; these might differ for TD affecting the systemic arterial (AT), systemic venous (VT), and pulmonary circulation (PT). Hypothesis/Objectives: To compare signalment and clinicopathological data between dogs with renal proteinuria with or without TD, and between dogs with AT, VT, and PT. Animals: One hundred fifty client-owned dogs with renal proteinuria, 50 of which had TD. Methods: Retrospective case-controlled study. A database search (2004-2021) identified proteinuric dogs (UPC>2) with and without TD. Clinicopathological data were obtained from the records. TD and non-TD (NTD) groups were compared by binary logistic regression, and AT, VT, and PT groups by multinomial regression. Normal data presented as mean ± SD, non-normal data presented as median [25th, 75th percentiles]. Results: Cavalier King Charles Spaniels [CKCS] were overrepresented in the TD group (OR=98.8, 95% CI 2.09-4671, P=.02). ... Our study identified that CKCS with renal proteinuria had higher rates of TD compared to other breeds. CKCS have been identified as predisposed to AT, particularly aortic thrombosis, although this has not been specifically associated with PLN. All 5 dogs of this breed with TD had AT (1 case had concurrent PT), 4 of which were aortic, although breed was not a significantly associated factor in the analysis of thrombus location. Reasons suggested for a genetic predisposition of CKCS for TD include a connective tissue disorder, a subset of the breed having increased platelet reactivity, and their increased incidence of cardiac disease. MMVD was present in 4 out of 5 of the CKCS with TD and was a common comorbidity in dogs of other breeds with TD in this study; however, the proportions of dogs with this comorbidity were not different between TD cases and NTD cases, and MMVD is not considered a risk factor for TD. ... Compared to NTD cases, TD cases had higher concentration of neutrophils (11.06 [8.92, 16.58]×109/L vs 7.31 [5.63, 11.06]×109/L, p=.02), and lower concentration of eosinophils (0 [0, 0.21]×109/L vs 0.17 [0.04, 0.41]×109/L, P=.002) in blood, and lower serum albumin (2.45±0.73g/dL vs 2.83±0.73g/dL, P=.04). AT cases had higher serum albumin concentrations than VT cases (2.73±0.48g/dL vs 2.17±0.49g/dL, P=.03) and were older than PT cases (10.6±2.6 years vs 7.0±4.3 years, P=.008). VT cases were older (9.1±4.2 years vs 7.0±4.3 years, P=.008) and had higher serum cholesterol concentration (398 [309-692 mg/dL] vs 255 [155-402 mg/dL], P=.03) than PT cases. Conclusions and Clinical Importance: Differences between thrombus locations could reflect differences in pathogenesis.
Detection of early renal perfusion changes by contrast-enhanced ultrasonography in dogswith preclinical myxomatous mitral valve disease. Saho Kamata, Tomoya Morita, Masahiro Yamasaki. Vet.Rad. & Ultrasound. November 2024; doi: 10.1111/vru.13459> Quote: Since the prognosis of myxomatous mitral valve disease (MMVD) varies, its charac- terization is clinically relevant, and renal impairment has been identified as one of its associated factors. Intrarenal Doppler ultrasonography (IRD), an intrarenal hemody- namic assessment method, is useful for predicting cardiac- and renal-related death but cannot detect early changes in dogs with preclinical MMVD. Contrast-enhanced ultrasonography (CEUS), another intrarenal hemodynamic assessment method, may identify earlier changes; however, renal perfusion evaluations using CEUS have not yet been performed on dogs with MMVD. We hypothesized that CEUS detects changes earlier than hout cardiac disease and preclinical MMVD dogs of different American College of Veterinary Internal Medicine stages using CEUS and compared it with IRD indices. Twenty-three dogs with MMVD (ten stage B1 and thir- teen stage B2) [4 cavalier King Charles spaniels (17.4%)] and 12 control dogs without cardiac disease were included. The rise times of the renal cortex and medulla were measured from a time-intensity curve. The rise time of the cortex was longer in dogs with stage B2 MMVD than in control dogs, while that of the medulla was shortened in the right ventricular dysfunction group in stage B2. No changes were observed in IRD indices (the resistance index and venous impedance index). In conclusion, CEUS detected changes in renal perfusion in dogs with preclinical MMVD even when IRD indices remained unchanged, suggesting the utility of CEUS in evaluations of renal perfusion in MMVD dogs.


 Cardio-Renal Syndrome
Cardio-Renal Syndrome
CONNECT WITH US