Arthritis (Osteoarthritis) and the
Cavalier King Charles Spaniel
-
 What It Is
What It Is - Symptoms
- Diagnosis
- Treatment
- What You Can Do
- Research News
- Related Links
- Veterinary Resources
Arthritis (osteoarthritis -- OA, or degenerative joint disease -- DJD) is the most commonly diagnosed joint disease in dogs.
In an April 2018 article, UK researchers examining all reported cases of arthritis in a UK dog population under primary veterinary care during 2013 found that 2.42% were treated for arthritis. This made the CKCS the 11th most frequently affected breed in the study. Of 10,143 cavaliers in the study, 245 were diagnosed with osteoarthritis. The most frequently affected breeds were: (1) golden retriever, (2) Labrador retriever, (3) Rottweiler, (4) German shepherd, and (5) border collie. The overall average of all dogs diagnosed with osteoarthritis was 2.5%.
See also our Immune-Mediated Polyarthritis (IMPA) webpage.
What It Is
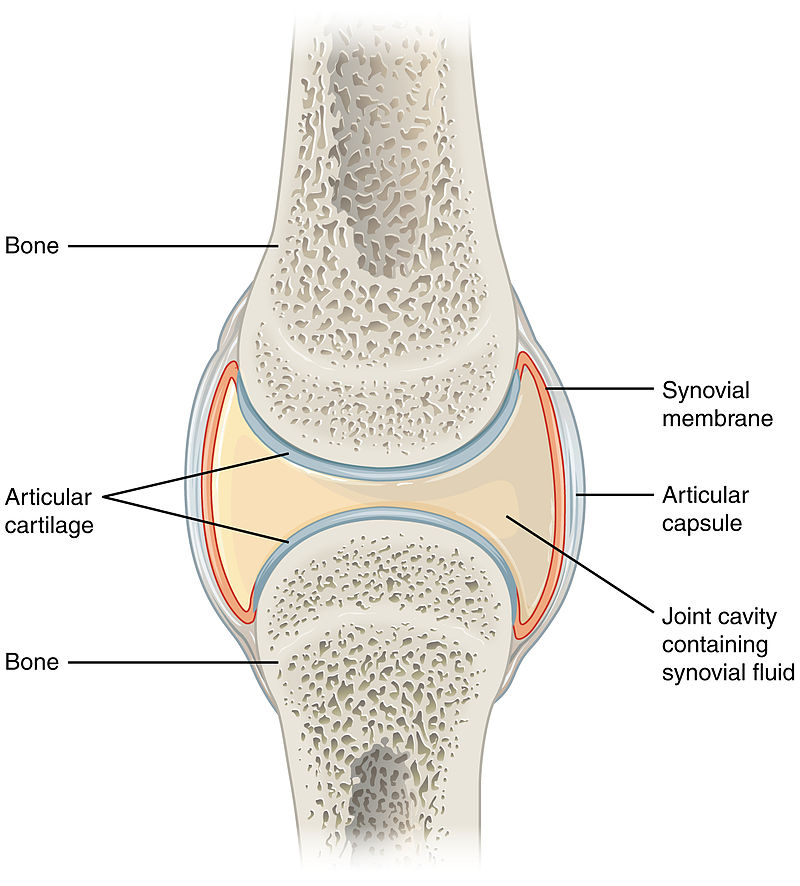
Arthritis is an incurable, progressive, degenerative disease within what are known as synovial joints, which are joints with a capsule filled with synovial fluid which surrounds the bones' surfaces. The degeneration leads to inflammation, impaired function, and pain. Arthritis is a multifactorial disease, primarily genetic but often exacerbated by the dog's diet and exercise levels. Arthritis often is secondary to a joint abnormality such as a ligament rupture, hip dysplasia, or patellar luxation. Arthritis may also be related to imbalances of the gastroinestinal (gut) microbiome -- dysbiosis.
Rapidly progressive osteoarthritis (RPOA) is a recent development, first reported around 2012 and attributed to OA treatments using anti-nerve growth factors (NGF). RPOA has been attributed to treating OA-affected dogs with bedinvetmab (Librela, Beransa), described in more depth below here.
An idiopathic form of arthritis, immune-mediated polyarthritis (IMPA), causing recurring episodes of lameness and joint swelling, has been reported in cavalier King Charles spaniels. See our Immune-Mediated Polyarthritis (IMPA) webpage.
RETURN TO TOP
Symptoms
The common signs that a dog suffers from OA are indications of pain when making certain movements or being in certain positions. Others include stiffness, limping, dereased activity, reluctance to play or jump, difficulty in getting up, lying down, jumping or climbing. More severe signs include clicking or popping sounds in joints, and muscle atrophy.
These signs tend to come in stages and progress. The early (mild) stage -- Stage 1 -- would include slight changes in mobility. The moderate stage -- Stage 2 -- would show progression to noticeable stiffness, pain, and limitations in activity. The severe (advanced) stage -- Stage 3 -- would be signs of severe pain, loss ofmobiliity, and secondary complications. The end stage -- Stage 4 -- would be pain so servere that the dog may not be able to walk on the affected leg, or able to walk at all.
RETURN TO TOP
Diagnosis
OA typically is diagnosed by physical examination of the affected joints, for pain, swelling, and range of motion, and gait and posture.
X-rays (radiographs) are essential to confirm OA, by showing joint swelling, spurs,, and changed shapes of the affected bones.
Other tests may include blood samples, joint fluid samples, and force plate analysis. It is very important to properly diagnose OA before prescribing certain medications, which are known to cause severe adverse reactions if administered to dogs which do not really have OA.
RETURN TO TOP
Treatment
- NSAIDs
- Adequan
- Amantadine
- Zydax
- Bedinvetmab (Librela)
- Mesotherapy
- Synovetin OA
- Krill Oil
- Palmitoylethanolamide (PEA)
- Nutraceuticals
- Cannabinol (CBD)
- Laser therapy
Since it is incurable, OA treatements intend to "manage" the disorder and try to make the patient as comfortable as possible. Keeping the affected dog lean and giving it appropriate daily exercise (leash walks and low-impact exercises) are important steps in reducing the progression of OA and its severity.
Medications which may be prescribed include NSAIDs (nonsteroidal anti-inflammatory drugs), which are designed to relieve pain and reduce inflammation. An NSAID growing in popularity for treating arthritis in dogs is grapiprant (Galliprant). A COX-2 selective NSAID, enflicoxib, has been found in a September 2021 study to be effective when administered orally. See, also, this February 2024 article regarding the safetly and efficacy of long-term use of enflicoxib. Other NSAIDs include Loxicom (Meloxicam). NSAIDs are known to have potentially harmful side effects, including gastrointestinal (vomiting, diarrhea, ulceration) and affecting kidney functions.
Polysulfated glycosaminoglycan (PSGAG) (Adequan) is injected into the muscles to relieve pain and inflammation and improve range of motion due to arthritis in dogs. Studies have found that it prevents catabolic enzymes from degrading cartilage and bone, the causes of arthritis. PSGAG has been approved by the US Food & Drug Administration (FDA) for the management of OA in dogs.
Amantadine (Endantadine, Gocovri, Osmolex ER, Symmetrel), is an N-methyl-d-aspartate antagonist (NMDA), which is used for control of the symptoms of Parkinson's disease in humans, together with gabapentin or pregabalin. Amantadine is believed to release brain dopamine from nerve endings making it more available to activate dopaminergic receptors. See this January 2008 article, in which the researchers found clinical improvement in dogs with chronic osteoarthritis pain when amantadine was used in combination with meloxicam. See Dr. Rusbridge's YouTube video on this drug.
Pentosan polysulfate sodium (Zydax) reportedly will reduce inflammation and slow the progression of arthritic damage to cartilage. It is injected every 4 weeks and has shown effectiveness within 6 weeks. Currently it is available for treating dogs only in New Zealand and Australia.
Bedinvetmab (Librela, Beransa) is a injectable solution to alleviate pain
associated with osteoarthritis (OA) in dogs. It is administered monthly.
It is a monoclonal antibody which binds to and targets
Nerve Growth
Factors (NGF), which have key involvement in OA pain. It is manufactured
by Zoetis. In May 2023, Zoetis announced that the USA Food & Drug
Administration approved Librela (bedinvetmab injection) for the control
of pain associated with osteoarthritis (OA) in dogs. Zoetis stated in
its press release that Librela is the first and only once-monthly,
anti-NGF monoclonal antibody treatment for canine OA pain and is
approved as
 safe and effective in providing long-term control of OA pain
symptoms in dogs, which can improve their mobility and overall quality
of life. Librela does not reduce inflammation or improve joint function.
It just masks feeling the pain associated with those conditions.
safe and effective in providing long-term control of OA pain
symptoms in dogs, which can improve their mobility and overall quality
of life. Librela does not reduce inflammation or improve joint function.
It just masks feeling the pain associated with those conditions.
Nevertheless, bedinvetmab is the subject of controversy due to reports of unanticipated adverse reactions, including ataxia, hind-end weakness, inability to walk, new or worsened seizures, pancreatitis, and organ damage. Among expressed concerns are that blocking NGF may prevent its known protective and reparative roles elsewhere in the body. See this May 2015 article and this October 2023 article.
Veterinary neurologist Dr. Curtis Dewey, who is conducting an investigation into the adverse reactions caused by bedinvetmab, makes this recommendation as of February 2025:
"This drug is for dogs with radiographically documented osteoarthritis (OA). This drug should not be administered to dogs with pre-existing neurologic disorders. All dogs for whom this drug is to be considered should have a neurologic examination performed first. In older dogs (9 years and up), a cognitive questionnaire should be completed by the pet parent to evaluate for potential cognitive dysfunction prior to injection."
See this May 2025 article in which reported:
"This study reveals a striking disparity in musculoskeletal adverse event reports to Librela compared to six comparator drugs. Ligament/tendon injuries, polyarthritis, fractures, musculoskeletal neoplasia, and septic arthritis were reported nine times more frequently in Librela-treated dogs. Worryingly, since its European release, Librela has accumulated 20 times more reports than the highest-ranking comparator drug (Rimadyl) and three times more than all comparator drugs combined over a 20-year period. Furthermore, independent expert review of a subset of cases strongly supported a causal association between Librela and accelerated joint destruction. ... Given NGF's diverse roles and prior evidence of RPOA, subchondral bone fractures, and atraumatic joint luxations in humans and animals, bedinvetmab-associated MSAEs are an expected consequence of NGF inhibition."
 See also this
July 2025 commentary on the May 2025 article, and this
August 2025 article. In an August 2025 post on Facebook by the
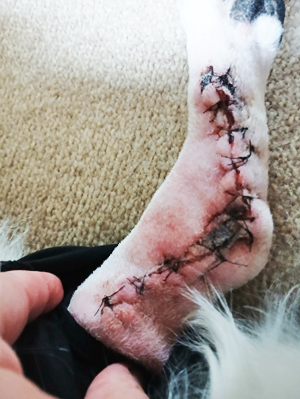
owner of a cavalier, Sam, she reported that he experienced rapidly progressive osteoarthritis (RPOA),
attributed to Librela and ended up having major arthrodesis (joint
fusion) surgery
(right) to remove his ankle joint and pin and plate his leg so that
he could walk again. Sam was originally prescribed Librela for
elbow dysplasia.
See also this
July 2025 commentary on the May 2025 article, and this
August 2025 article. In an August 2025 post on Facebook by the
owner of a cavalier, Sam, she reported that he experienced rapidly progressive osteoarthritis (RPOA),
attributed to Librela and ended up having major arthrodesis (joint
fusion) surgery
(right) to remove his ankle joint and pin and plate his leg so that
he could walk again. Sam was originally prescribed Librela for
elbow dysplasia.
Before even considering having an arthritic dog injected with bedinvetmab (Librela, Beransa), check out the current information on the Pet Advocate website about this drug.
Mesotherapy, also known as local intradermal therapy, is a technique of injecting medications and supplements into the dog's dermis, which is the middle layer of the skin. In a January 2023 article, 20 police dogs diagnosed with OA were treated by mesoherapy with a combination of lidocaine, piroxicam, and thiocolchicoside, injected in intradermal points. A control group of 10 other affected dogs were treated with the NSAID meloxicam. The researchers reported that the mesotherapy protocol reduced pain scores and other clinical measurement scores lasting for longer periods than the meloxicam group.
Synovetin OA is a "conversion electron therapeutic veterinary device" which is injected into the dog's elbow joints which are afflicted with osteoarthritis to reduce pain and inflammation of the synovial membrane which lines the elbow (synovitis).
Krill oil has antioxidant and anti-inflammatory effects of omega-3 polyunsaturated fatty acids (PUFAs). It has been found to provide pain relief in dogs with OA without any adverse effects. Strong scientific evidence has been found to suport the use of omega-3 fatty acids, in general, to alleviate the signs of OA.
Palmitoylethanolamide (PEA)
is a
N-acylethanolamine molecule in a family of long-chain fatty acid
 amides
called ALIAmides. PEA has been found in rat and mice studies to limit
hyperactvity in immune cells and thereby control inflammatory responses
and resulting tissue damage. PEA is produced by the animal's body as
needed in response to certain types of injuries. PEA is a product of
normal fatty acid synthesis from palmitic acid. It is found in many
common foods, particularly palm oil, soy beans, egg yolks, and peanuts.
The commercial version is most commonly manufactured from palm oil*.
amides
called ALIAmides. PEA has been found in rat and mice studies to limit
hyperactvity in immune cells and thereby control inflammatory responses
and resulting tissue damage. PEA is produced by the animal's body as
needed in response to certain types of injuries. PEA is a product of
normal fatty acid synthesis from palmitic acid. It is found in many
common foods, particularly palm oil, soy beans, egg yolks, and peanuts.
The commercial version is most commonly manufactured from palm oil*.
Not all PEA is alike. There are at least 4 types of PEA, and the differences of those 4 are described below here. The differences are mainly the sizes of the particles. The larger the size of particle, the less soluble and less bioavailable. Micronization reduces the size of the particles of PEA. In short, since the basic (naïve) PEA is almost totally insoluble in water and therefore has very poor bioavailability, researchers use micronized or ultra-micronized PEA or water-dispersible PEA in their published studies.
• Basic PEA, called "naïve PEA", is almost totally insoluble in water and under gastrointestinal conditions and therefore the oral intake of it (rather than being injected directly into the abdomen) has very poor bioavailability, meaning that it does not get absorbed well in the dog's gut. See this May 2021 article and this July 2025 article.
• Micronized PEA (m-PEA or micro-palmitoylethanolamide) is a patented technique that reduces the diameter of PEA particles, making them absorbable in the intestine, which has been found to be more effective than ordinary basic PEA in activating PEA levels in blood plasma in dogs. See this August 2014 article.
• Ultra-micronized PEA (um-PEA), also patented, reduces the PEA particle size further, to enable it to cross the blood-brain barrier, likewise has been found to be much more effective than basic PEA. See this August 2014 article.
• Water-Dispersible PEA (PEA-WD), also patented, reduces the PEA to a powder which can be dispersed in cold water. It has been found to be 16 times more effective than basic PEA. See this July 2025 article.
• Hybrid versions of PEA: Additionally, PEA has been combined with other ingredients and used in some published studies. These include FenuMat-PEA (P-fen), which is a PEA hybrid combined with the herb fenugreek (trigonella foenum-graecum), and hybrids combined with resveratrol, quercetin, fisstin, and boswellic acid. See this July 2025 article.
PEA micronization and ultra-micronization are patented (by Italian company, EPITECH Group SpA) processing techniques that reduce the diameter of the PEA particle to a micronized or ultra-micronized size which optimizes the PEA's absorbability along the intestine. Micronization increases the drug's surface area, thereby improving its dissolution rate and minimizing its absorption difficulties. The ultra-micronized size also enables the PEA to cross the blood-brain barrier. See this February 2021 article.
 If a PEA product is not advertised as being micronized or
ultra-micronized, then
Dr. Clare Rusbridge advises that
"You
probably are wasting your money."
A variety of brands of
micronized and ultra-micronized PEA are offered on-line.
If a PEA product is not advertised as being micronized or
ultra-micronized, then
Dr. Clare Rusbridge advises that
"You
probably are wasting your money."
A variety of brands of
micronized and ultra-micronized PEA are offered on-line.
As for dosages, the studies using micronized PEA, the range was from 10 to 15 mg/kg/day, and the range for ultra-micronized was 24 mg/kg (for osteoarthritis in humans).
There are no published veterinary journal articles about treating canines with PEA for osteoarthritis (OA). This is very limited research regarding a combination of ultramicronized PEA (um-PEA) and quercetin (PEA-Q), first in rats and finally in dogs.
The rat research has been an August 2017 article. The authors report that a combination of ultramicronized PEA and quercetin (PEA-Q), given orally to 10 rats. They reported:
"PEA-Q is a novel co-ultramicronized formulation of PEA and quercetin whose effects were investigated in two pre-clinical models of OA pain in rats. Oral administration of PEA-Q decreased pain sensitivity, improved locomotor function, reduced inflammatory signs and mediators and lowered histological damage score."
In that article, the authors go to great lengths to suggest that their "collective observations presented here propose that PEA-Q shows promise for multimodal pain management in canine and feline OA."
In a September 2018 report, 13 dogs diagnosed with chronic OA and lameness received supplements of PEA-Q for four weeks, at the rate of 24 mg/kg per day. Pain scores were taken, showing successful improvement in 7 of the dogs by the second week. Lameness also reportedly improved durint the treatment period.
Nevertheless, at least one PEA vendor advertises that PEA alone (meaning, not even ultramicronized PEA and not combined with quercetin) "may be beneficial for ... Osteoarthritis pain". So, Buyer Beware. See more about this type of hazard at this link.
PEA's fellow ALIAmide, micronized palmitoyl-glucosamine (m-PGA), has been teamed with curcumin (m-PGA-Cur) to treat dogs with osteoarthritis. In an October 2020 report of 181 dogs diagnosed with OA, they were given m-PGA-Cur for 2 months in addition to their conventional medications. The dogs were scored for lameness and pain at the start of the study and each 30 days thereafter, along with having the dogs' owners evaluate their dogs' mobility impairment and pain behaviors. At the end of the study, 74% of the owners scored their dogs' quality of life as improved, and 87% of the 27 participating veterinarians expressed satisfaction with the addition of m-PGA-Cur in the daily management of the OA. This was not a controlled clinical trial, however.
In a February 2023 article, m-PGA and curcumin again were teamed (m-PGA-Cur) to treat 58 dogs having chronic OA pain. All of the dogs had been treated with meloxicam and continued to do so during the 18-week testing period. The aim of the study was to determine if adding m-PGA-Cur to the treatment regime enabled the meloxicam to be tapered at the rate of 25% during the first 8 weeks of combined treatment without experiencing worsening of pain. The investigators found that 75% of the dogs were assessed as having no pain increases 10 weeks after the withdrawal of meloxicam. They concluded that the m-PGA-Cur was able to maintain the level of pain relief previously maintained only by the meloxicam.
Read more about PEA on our PEA webpage.
* Palm oil: The palm oil cultivation industry has been destroying rainforests in Sumatra and Borneo in Indonesia and Malaysia, the only habitats of orangutans. If you are going to obtain PEA, we suggest that you do so only from vendors whose PEA has been manufactured with palm oil from sustainable sources and not the deforestation of rainforests. Read more about finidng sources of sustainable palm oil at www.orangutanlandtrust.com. To avoid palm oil as the source of PEA, read the ingredients descriptions on the brands of PEA in which you are interested.
Nutraceuticals are nutrients necessary for
supporting or improving normal structure and function of the
 weight-bearing joints. They have been found to:
weight-bearing joints. They have been found to:
• Support or enhance metabolism of cartilage cells (chondrocyte) and joint membranes (synoviocyte) -- an anabolic effect;
• Inhibit damaging enzymes within synovial fluid and cartilage -- a catabolic effect;
• Inhibit formation of thrombi in small blood vessels supplying the joint (antithrombolic).
The most common nutraceuticals are glucosamine and chondroitin. Glucosamine regulates the synthesis of collagen in cartilage, and may provide mild anit-inflammatory effects. Crystalline glucosamine sulphate has the greatest efficacy and bioavailablity for osteoarthritis. Chondroitin sulfate inhibits destructive enzymes in joint fluid and cartilage. Both of them also contribute to the building blocks synthesis of glycoaminoglycans and proteoglycans) for the formation and repair of cartilage.
Bioactive Collagen Peptides (BCP) are a nutraceutical intended to structurally improve affected tissues and slow disease progression. Collagen-derived peptides have been shown to accumulate in cartilage tissue, where they can stimulate chondrocytes to synthesize extracellular cartilage matrix molecules and counteract progressive tissue degeneration.
Undenatured type-II collagen (UC-II), a patented form of collagen extracted from the cartilage of the chicken's sternum, has been found effective in reducing pain and discomfort experienced by dogs suffering from osteoarthritis.
One study showed that undenatured Type II collagen decreased inflammation in healthy young dogs following strenuous exercise (72). In dogs with OA, two clinical trials investigated the effects of undenatured Type II collagen using objective measures of gait. Decreased pain was observed in these trials (73, 74). The positive impact of undenatured Type II collagen in dogs with OA appears to be dose dependent, with a stronger response noted in dogs receiving 80 mg per day than 40 mg per day.
Microlactin (Duralactin) is a nutraceutical consisting of a protein concentrate derived from the milk of hyper-immunized cows, called hyper-immune milk factor (HIMF). It reportedly impedes inflammation and can be used safely in dogs. It is slow to respond to pain, taking a week to two weeks for maximum effectiveness.
 Cannabinol (CBD)
is
is a cannabinoid compound produced from hemp and marijuana (Cannabis
Sativa) plants. CBD oil mimics the endocannabinoid molecules which the
dog's (and our) body produces in several different organs. They play
roles in reducing pain, regulating inflammation, and affecting the
immune system, by initially binding to receptors in the brain.
Cannabinol (CBD)
is
is a cannabinoid compound produced from hemp and marijuana (Cannabis
Sativa) plants. CBD oil mimics the endocannabinoid molecules which the
dog's (and our) body produces in several different organs. They play
roles in reducing pain, regulating inflammation, and affecting the
immune system, by initially binding to receptors in the brain.
CBD is non-psychoactive, unlike tetrahydrocannabinol (THC), another cannabinoid compound from marijuana, which is considered psychoactive by altering the mental state, and can be highly toxic to dogs.
Varieties of CBD: Cannabidiol-based veterinary products are derived mainly from hemp (Cannabis sativa) and must contain less than 0.3% tetrahydrocannabinol (THC). This form of CBD can be processed into "full spectrum" or "broad spectrum" and also may be in the form of a "distillate", in which all THC has been removed, or in the form of CBD "isolate", which is a purifed powder.
• Full Spectrum: Full spectrum CBD contains other extracts found in the cannabis plant, including terpenes, and up to 0.3% THC.
• Broad Spectrum: Broad spectrum CBD also contains some other cannabis compounds but no more than trace amounts of THC.
• CBD Isolate: CBD isolate is pure CBD and contains no other cannabis plant compounds.
• Naked CBD: Naked CBD describes CBD oil by itself, as opposed to being capsultated or microcapsulated or combined with any other substance, such as deoxycholic acid (DCA).
• Liposomal CBD: This is an orally administered encapsultated CBD which is packaged within liposomes, small fatty cellular sacs which improve bioavailability of the CBD by enabling it to be withstand digesstion in the stomach and degradation in the liver. Lipsomal CBD was tested on dogs in this September 2020 article.
• Cannabidiolic acid (CBDA) is an acid precursor of CBD. It forms CBD when heated. It has been shown in some studies to be more potent that CBD for treating rats. It has been found to be more readily absorbed into the human bloodstream than CBD. Aa theory is that adding CBDA to doses of CBD may make the CBD more absorbable. In this September 2020 article, the investigators found that CBDA is absorbed at least twice as well as CBD in dogs within a 24 hour period, with some differences depending upon the medium used to deliver the oral treatment.
 The
most canine studies of CBD thus far have been regarding
osteoarthritis (OA). In the only study thus far which included cavalier
King Charles spaniels, the
March 2020 article, the investigators found that the only breed
which did not show any improvement after being treated with CBD at the
highest dose, was the cavalier breed. Otherwise, on a species-wide
basis, the results have been fairly favorable, with the
clear exception of one study, the one reported in the
March 2021 article.
The
most canine studies of CBD thus far have been regarding
osteoarthritis (OA). In the only study thus far which included cavalier
King Charles spaniels, the
March 2020 article, the investigators found that the only breed
which did not show any improvement after being treated with CBD at the
highest dose, was the cavalier breed. Otherwise, on a species-wide
basis, the results have been fairly favorable, with the
clear exception of one study, the one reported in the
March 2021 article.
In a July 2018 article, researchers report on a study of 16 dogs to determine the safety of CBD extract and its efficacy in allevieating pain in dogs diagnosed with osteoarthritis (OA). The equal mix of CBD and carboxylic acid of CBD (CBDA), was administered at 2mg/kg every 12 hours for 4 weeks. The treated dogs were:
"... perceived to be more comfortable and active. There appear to be no observed side effects of the treatment ... dogs undergoing OA treatment for a month duration. There were some dogs with incidental rises in alkaline phosphatase that could be related to the treatment. Further long-term studies with larger populations are needed to identify long-term effects of CBD rich industrial hemp treatment, however short term effects appear to be positive."
In a March 2020 article about a pilot study of 32 osteoarthritic dogs, in which two cavalier King Charles spaniels were included, hemp-derived CBD oil administered to the dogs reportedly "appears to positively affect dogs with chronic maladaptive pain by decreasing their pain, thereby improving their mobility and quality of life." The specific ingredients of the CBD oil and its dosages were:
"At the initial evaluation and enrollment, qualified dogs received a CBD oil product at a dose of 0.25 mg/kg delivered on food QD for 3 days and then morning and night (approximately every 12 hours). The product given was a certified organic, cold-pressed hemp seed oil infused with 1,000 mg of full-spectrum hemp extract derived from organically grown hemp plants, cultivated in Colorado. Full-spectrum extract includes cannabinoids (such as cannabidiolic acid, CBD, cannabigerol, cannabichromene), flavonoids, terpenes, and other constituents within the cannabis plant."
Most interestingly, the investigators singled out the two CKCSs:
"Among these 30 dogs, the dose of CBD needed to achieve a positive effect ranged from 0.3 up to 4.12 mg/kg BID. The 2 dogs in the study requiring the highest dose of the CBD product were both Cavalier King Charles spaniels (not related to one another), and neither of these dogs experienced any changes/elevations in liver enzymes."
In an August 2020 article, 9 dogs being treated for chronic osteoarthritis-related pain with conventional medications were dosed with oral transmucosal (OTM) cannabidiol (CBD) (2 mg/kg) every 12 hours for 12 weeks. The investigators report that the Pain Severity Score and the Pain Interference Score were significantly lower in CBD than in the control group of 12 other dogs, and the Quality of Life Index was significantly higher in the CBD group. They concluded:
In an August 2020 article, 9 dogs being treated for chronic osteoarthritis-related pain with conventional medications were dosed with oral transmucosal (OTM) cannabidiol (CBD) (2 mg/kg) every 12 hours for 12 weeks. The investigators report that the Pain Severity Score and the Pain Interference Score were significantly lower in CBD than in the control group of 12 other dogs, and the Quality of Life Index was significantly higher in the CBD group. They concluded:
"The addition of OTM CBD showed promising results. Further pharmacokinetics and long-term studies in larger populations are needed to encourage its inclusion into a multimodal pharmacological approach for canine osteoarthritis-related pain."
In a September 2020 article, 15 dogs diagnosed with osteoarthritis were divided into 3 groups and administered (a) 20 mg/day (0.5 mg/kg) naked CBD, (b) 50 mg/day (1.2 mg/kg) naked CBD, or (c) 20 mg/day liposomal CBD for 30 days. The dogs were tested on the first and last days for four different movements: sitting to standing, lying to standing, walking, and running. The investigators report that, among the 5 dogs receiving 20 mg/day of naked CBD, there generally were no improvements noted among all four movement categories. As for the 5 dogs being dosed 50 mg/day, there were "significant improvements" among all four assessment categories, as also were the 5 dogs receiving 20 mg/day liposomal CBD.
In a March 2021 article, 23 dogs with naturally occurring osteoarthritis of appendicular joints received 2.5 mg/kg of a CBD isolate in hempseed oil for six weeks. The investigators measured outcome objective gait analysis, activity counts (via accelerometry) and clinical metrology instruments. They report finding no differences noted between the CBD group and the placebo group at any time point for any of the recorded outcome measures. As adverse events, then noted elevation in liver enzymes in a majority of the dogs, and vomiting in two. They concluded that, "The pilot data from this study do not support the use of CBD as a symptom-relieving agent for canine OA."
In an August 2024 article, Thai researchers studied the effect of a combination of CBD and krill oil in providing pain relieve to dogs (none being cavaliers) dignosed with grade II chronic lameness due to stifle (knee of the hind leg) OA. The combination was in the form of a biscuit made by the corporate sponsor of the study. Thirty dogs were in the study, with 10 in a placebo group, 10 in a krill oil only group, and 10 in the group which combined krill oil and CBD. The treatment goal was to decrease pain levels and inflammation. After 28 days, the researcheres found no statistically significant difference in the pain interference scores (PIS) and pain severity scores (PSS) between the krill oil and CBD + krill oil groups. Also, the stifle temperature of the three groups at different periods did not significantly differ. They concluded that they found no clear data supporting the clinical value of adding CBD to krill oil.
In a March 2025 article reviewing the previous studies, the authors emphasized two main points:
(1) "The evidence supporting its use as an adjuvant to conventional therapy remains weak. Further studies utilising objective measurements are needed to improve the strength of the supporting evidence for a general use of CBD oil as additional analgesia for dogs with OA."
(2) "It was described in Kogan et al. (2020) that there were 2 'non-responders' amongst the 32 dogs who did not show any changes to overall mobility and comfort during the study, with their overall pain scores remaining at 1/10 despite the fact that the CBD dose was increased along the course of the 90-day study. It is uncertain if it coincidental or a breed-related response as both 'non-responders' were [Cavalier] King Charles Spaniels. This breed of dog was not included in the other three reviewed papers (Brioschi et al., 2020 ; Gamble et al., 2018; Mejia et al., 2021), disallowing further inquiry into the response of CBD in the breed."
See our Cannabis webpage for additional details about CBD, including delivery methods, bioavailability, dosages, and adverse reactions.
Laser therapy, called "photobiomodulation" (PBM), which is continuous wave laser treatments at acupuncture sites, is a treatment option, either with or without medication.
RETURN TO TOP
What You Can Do
Plan ahead. Assume your dog will develop (or already is developing) OA, and take these steps:
• Excercise your dog daily, under controlled conditions, especially walks on leash and harness. Over-doing exercise can worsen the condition of OA-affected dogs. So, walk but don't run. Shorter walks and not longer ones.
• Manage your dog's weight. Obesity can aggravate OA and cause it to progress. Owners should avoid over-feeding and under-exercising their cavaliers. See this section of our Diets Webpage on Body Condtion Scoring to learn how much your cavalier should weigh.
• Physical rehabilitation exercises, such as massages and stretching.
• Dietary supplements, especially omega-3 fatty acids, such as fish oil or sardines.
 Owners may provide their affected dogs with supplements such as
glucoamine HCI, methylsulfonylmethane (MSM), N,N-Dimethylglycine HCI
(DMG), and manganese. Retail combinations of these supplements include
Vetri-Science GlycoFlex Joint Support.
Owners may provide their affected dogs with supplements such as
glucoamine HCI, methylsulfonylmethane (MSM), N,N-Dimethylglycine HCI
(DMG), and manganese. Retail combinations of these supplements include
Vetri-Science GlycoFlex Joint Support.
An aid to cavaliers suffering from arthritis is an elevated dog food
bowl. The
Ergo Feeder
(above right) is an
 example.
example.
Read more about canine arthritis on the Canine Arthritis Management website.
RETURN TO TOP
Research News
April 2018:
Cavaliers rank 11th in diagnosis of arthritis in UK 2013 study
of most frequently affected breeds.
 In
an
April 2018 article, UK researchers (Katharine L. Anderson, Dan G.
O'Neill, David C. Brodbelt, David B. Church, Richard L. Meeson, David
Sargan, Jennifer F. Summers, Helen Zulch, Lisa M. Collins [right])
examining all reported cases of osteoarthritis in a UK dog population
under primary veterinary care during 2013, found that 2.42% were
diagnosed with osteoarthritis. This made the CKCS the 11th most
frequently affected breed in the study. Of 10,143 cavaliers in the
study, 245 were diagnosed with osteoarthritis. The most frequently
affected breeds were: (1) golden retriever, (2) Labrador retriever, (3)
Rottweiler, (4) German shepherd, and (5) border collie. The overall
average of all dogs diagnosed with osteoarthritis was 2.5%.
In
an
April 2018 article, UK researchers (Katharine L. Anderson, Dan G.
O'Neill, David C. Brodbelt, David B. Church, Richard L. Meeson, David
Sargan, Jennifer F. Summers, Helen Zulch, Lisa M. Collins [right])
examining all reported cases of osteoarthritis in a UK dog population
under primary veterinary care during 2013, found that 2.42% were
diagnosed with osteoarthritis. This made the CKCS the 11th most
frequently affected breed in the study. Of 10,143 cavaliers in the
study, 245 were diagnosed with osteoarthritis. The most frequently
affected breeds were: (1) golden retriever, (2) Labrador retriever, (3)
Rottweiler, (4) German shepherd, and (5) border collie. The overall
average of all dogs diagnosed with osteoarthritis was 2.5%.
November 2016:
Cavaliers were 15+% of dogs with erosive immune-mediated
polyarthritis (IMPA).
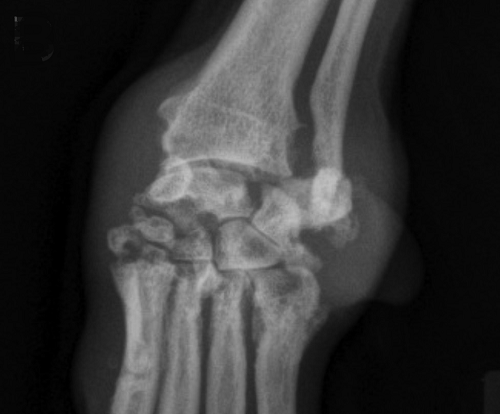 In
a
November 2016 article, a team of Wisconsin and Michigan researchers
(Magen L. Shaughnessy, Susannah J. Sample, Carter Abicht, Caitlin
Heaton, Peter Muir) studied the clinical records of 79 dogs
diagnosed with immune-mediated polyarthritis (IMPA). Of those 79 dogs,
13 had erosive IMPA, of which, two were cavalier King Charles spaniels.
The 13 affected dogs had erosive lesions in their carpal joints. The
estimated median synovial fluid lymphocyte count for dogs with erosive
IMPA was significantly greater than that for dogs with nonerosive IMPA.
Results indicated erosive IMPA most commonly affected the carpal joints
of middle-aged small-breed dogs. (See left carpus of an 8-year-old
spayed female Cavalier King Charles Spaniel with subjectively moderate
subchondral bone lysis accompanied by severe synovial swelling -- in
x-ray at right.)
In
a
November 2016 article, a team of Wisconsin and Michigan researchers
(Magen L. Shaughnessy, Susannah J. Sample, Carter Abicht, Caitlin
Heaton, Peter Muir) studied the clinical records of 79 dogs
diagnosed with immune-mediated polyarthritis (IMPA). Of those 79 dogs,
13 had erosive IMPA, of which, two were cavalier King Charles spaniels.
The 13 affected dogs had erosive lesions in their carpal joints. The
estimated median synovial fluid lymphocyte count for dogs with erosive
IMPA was significantly greater than that for dogs with nonerosive IMPA.
Results indicated erosive IMPA most commonly affected the carpal joints
of middle-aged small-breed dogs. (See left carpus of an 8-year-old
spayed female Cavalier King Charles Spaniel with subjectively moderate
subchondral bone lysis accompanied by severe synovial swelling -- in
x-ray at right.)
RETURN TO TOP
RETURN TO TOP
Veterinary Resources
Idiopathic, non-infectious, non-erosive arthritis in a bitch. C.R. Hutchings, G.A. Verkerk, K.J. Kissling. New Zealand Vet. J. 1980;28(5). Quote: A 2 1/2-year-old Cavalier King Charles Spaniel bitch was examined because of recurring episodes of lameness and joint swelling over a period of 2 years. Clinical examination, radiography, serology, joint-fluid analysis and histopathology of the joint capsule led to a diagnosis of idiopathic, non-infectious, non-erosive arthritis. Combination therapy with prednisolone, cyclophosphamide and azothioprine produced a rapid improvement in the dog's condition. The features of this disease and its relationship to rheumatoid arthritis and systemic lupus erythematosis are discussed.
Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. Roush JK, Cross AR, Renberg WC, Dodd CE, Sixby KA, Fritsch DA, et al. J. Am. Vet. Med. Assn. Jan. 2010; doi: 10.2460/javma.236.1.67. Quote: Objective: To evaluate the effects of a food supplemented with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. Design: Randomized, double-blinded, controlled clinical trial. Animals: 38 client-owned dogs with osteoarthritis examined at 2 university veterinary clinics. Procedures: Dogs were randomly assigned to receive a typical commercial food (n = 16) or a test food (22) containing 3.5% fish oil omega-3 fatty acids. On day 0 (before the trial began) and days 45 and 90 after the trial began, investigators conducted orthopedic evaluations and force-plate analyses of the most severely affected limb of each dog, and owners completed questionnaires to characterize their dogs' arthritis signs. Results: The change in mean peak vertical force between days 90 and 0 was significant for the test-food group (5.6%) but not for the control-food group (0.4%). Improvement in peak vertical force values was evident in 82% of the dogs in the test-food group, compared with 38% of the dogs in the control-food group. In addition, according to investigators' subjective evaluations, dogs fed the test food had significant improvements in lameness and weight bearing on day 90, compared with measurements obtained on day 0. Conclusions and Clinical Relevance: At least in the short term, dietary supplementation with fish oil omega-3 fatty acids resulted in an improvement in weight bearing in dogs with osteoarthritis.
Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. Roush JK, Dodd CE, Fritsch DA, Allen TA, Jewell DE, Schoenherr WD, et al. J. Am. Vet. Med. Assn. January 2010; doi: 10.2460/javma.236.1.59. Quote: Objective: To assess the effect of food containing high concentrations of fish oil omega-3 fatty acids and a low omega-6-omega-3 fatty acid ratio on clinical signs of osteoarthritis in dogs. Design: Randomized, double-blinded, controlled clinical trial. Animals: 127 client-owned dogs with osteoarthritis in 1 or more joints from 18 privately owned veterinary clinics. Procedures: Dogs were randomly assigned to be fed for 6 months with a typical commercial food or a test food containing a 31-fold increase in total omega-3 fatty acid content and a 34-fold decrease in omega-6-omega-3 ratio, compared with the control food. Dog owners completed a questionnaire about their dog's arthritic condition, and investigators performed a physical examination and collected samples for a CBC and serum biochemical analyses (including measurement of fatty acids concentration) at the onset of the study and at 6, 12, and 24 weeks afterward. Results: Dogs fed the test food had a significantly higher serum concentration of total omega-3 fatty acids and a significantly lower serum concentration of arachidonic acid at 6, 12, and 24 weeks. According to owners, dogs fed the test food had a significantly improved ability to rise from a resting position and play at 6 weeks and improved ability to walk at 12 and 24 weeks, compared with control dogs. Conclusions and Clinical Relevance: Ingestion of the test food raised blood concentrations of omega-3 fatty acids and appeared to improve the arthritic condition in pet dogs with osteoarthritis.
Progressive lameness in a Cavalier King Charles Spaniel. Mark Longley, Kinley Smith. BSAVA Companion. April 2011; doi: 10.22233/20412495.0411.10. Quote: An 8-year-old Cavalier King Charles Spaniel presented with a 6-month history of progressive forelimb lameness. The diagnosis and treatment of rheumatoid arthritis is described.
Comparative therapeutic efficacy and safety of type-II collagen (UC-II), glucosamine and chondroitin in arthritic dogs: pain evaluation by ground force plate. Gupta RC, Canerdy TD, Lindley J, Konemann M, Minniear J, Carroll BA, et al. J. Anim. Physiol. Anim. Nutr. (Berl). May 2011; doi: 10.1111/j.1439-0396.2011.01166.x. Quote: The investigation was conducted on client-owned moderately arthritic dogs with two objectives: (i) to evaluate therapeutic efficacy of type-II collagen (UC-II) alone or in combination with glucosamine hydrochloride (GLU) and chondroitin sulphate (CHO), and (ii) to determine their tolerability and safety. Dogs in four groups (n = 7-10), were treated daily for a period of 150 days with placebo (Group-I), 10 mg active UC-II (Group-II), 2000 mg GLU + 1600 mg CHO (Group-III), and UC-II + GLU + CHO (Group-IV). On a monthly basis, dogs were evaluated for observational pain (overall pain, pain upon limb manipulation, and pain after physical exertion) using different numeric scales. Pain level was also measured objectively using piezoelectric sensor-based GFP for peak vertical force and impulse area. Dogs were also examined every month for physical, hepatic (ALP, ALT and bilirubin) and renal (BUN and creatinine) functions. Based on observations, significant (p < 0.05) reduction in pain was noted in Group-II, III, and IV dogs. Using GFP, significant increases in peak vertical force (N/kg body wt) and impulse area (N s/kg body wt), indicative of a decrease in arthritis associated pain, were observed in Group-II dogs only. None of the dogs in any group showed changes in physical, hepatic or renal functions. In conclusion, based on GFP data, moderately arthritic dogs treated with UC-II (10 mg) showed a marked reduction in arthritic pain with maximum improvement by day 150. UC-II, GLU and CHO operate through different mechanisms of action, and were well tolerated over a period of 150 days.
Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. Daniela Impellizzeri, Giuseppe Bruschetta, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Emanuela Esposito, Salvatore Cuzzocrea. Neuroinflammation. August 2014; doi: 10.1186/s12974-014-0136-0. Quote: Background: The fatty acid amide palmitoylethanolamide (PEA) has been studied extensively for its anti-inflammatory and neuroprotective actions. The lipidic nature and large particle size of PEA in the native state may limit its solubility and bioavailability when given orally, however. Micronized formulations of a drug enhance its rate of dissolution and reduce variability of absorption when orally administered. The present study was thus designed to evaluate the oral anti-inflammatory efficacy of micronized/ultramicronized versus nonmicronized PEA formulations. Methods: Micronized/ultramicronized PEA was produced by the air-jet milling technique, and the various PEA preparations were subjected to physicochemical characterization to determine particle size distribution and purity. Each PEA formulation was then assessed for its anti-inflammatory effects when given orally in the carrageenan-induced rat paw model of inflammation, a well-established paradigm of edema formation and thermal hyperalgesia. Results: Intraplantar injection of carrageenan into the right hind paw led to a marked accumulation of infiltrating inflammatory cells and increased myeloperoxidase activity. Both parameters were significantly decreased by orally given micronized PEA (PEA-m; 10 mg/kg) or ultramicronized PEA (PEA-um; 10 mg/kg), but not nonmicronized PeaPure (10 mg/kg). Further, carrageenan-induced paw edema and thermal hyperalgesia were markedly and significantly reduced by oral treatment with micronized PEA-m and ultramicronized PEA-um at each time point compared to nonmicronized PeaPure. However, when given by the intraperitoneal route, all PEA formulations proved effective. Conclusions: These findings illustrate the superior anti-inflammatory action exerted by orally administered, micronized PEA-m and ultramicronized PEA-um, versus that of nonmicronized PeaPure, in the rat paw carrageenan model of inflammatory pain.
Nerve Growth Factor: A Focus on Neuroscience and Therapy. Luigi Aloe, Maria Luisa Rocco, Bijorn Omar Balzamino, Alessandra Micera. Curr. Neuropharmacol. May 2015; doi: 10.2174/2F1570159X13666150403231920 Quote: Nerve growth factor (NGF) is the firstly discovered and best characterized neurotrophic factor, known to play a critical protective role in the development and survival of sympathetic, sensory and forebrain cholinergic neurons. NGF promotes neuritis outgrowth both in vivo and in vitro and nerve cell recovery after ischemic, surgical or chemical injuries. Recently, the therapeutic property of NGF has been demonstrated on human cutaneous and corneal ulcers, pressure ulcer, glaucoma, maculopathy and retinitis pigmentosa. NGF eye drops administration is well tolerated, with no detectable clinical evidence of systemic or local adverse effects. The aim of this review is to summarize these biological properties and the potential clinical development of NGF.
Clinical features and pathological joint changes in dogs with
erosive immune-mediated polyarthritis: 13 cases (2004-2012).
Magen L. Shaughnessy, Susannah J. Sample, Carter Abicht, Caitlin
Heaton, Peter Muir. JAVMA. November 2016;249(10):1156-1164. Quote:
Objective: To evaluate the clinical features and pathological joint
changes in dogs with erosive immune-mediated polyarthritis (IMPA).
Design: Retrospective case series. Animals: 13 dogs with erosive
IMPA [including two cavalier King Charles spaniels
(15.4%)] and 66 dogs with nonerosive IMPA. Procedures: The medical
record database of a veterinary teaching hospital was reviewed to
identify dogs with IMPA that were examined between October 2004 and
December 2012.
 For
each IMPA-affected dog, information extracted from the medical
record included signalment, diagnostic test results, radiographic
findings, and treatments administered. Dogs were classified as
having erosive IMPA if review of radiographs revealed the presence
of bone lysis in multiple joints, and descriptive data were
generated for those dogs. All available direct smears of synovial
fluid samples underwent cytologic evaluation. The synovial fluid
total nucleated cell count and WBC differential count were estimated
and compared between dogs with erosive IMPA and dogs with nonerosive
IMPA. Results: 13 of 79 (16%) dogs had erosive IMPA. Dogs with
erosive IMPA had a mean ± SD age of 7.1 ± 2.4 years and body weight
of 8.3 ± 3.4 kg (18.3 ± 7.5 lb). All 13 dogs had erosive lesions in
their carpal joints. (See left carpus of an 8-year-old spayed
female Cavalier King Charles Spaniel with subjectively moderate
subchondral bone lysis accompanied by severe synovial swelling -- in
x-ray at right).The estimated median synovial fluid lymphocyte
count for dogs with erosive IMPA was significantly greater than that
for dogs with nonerosive IMPA. All dogs received immunosuppressive
therapy with leflunomide (n = 9), prednisone (3), or
prednisone-azathioprine (1). Conclusions & Clinical Relevance:
Results indicated erosive IMPA most commonly affected the carpal
joints of middle-aged small-breed dogs. Further genetic analyses and
analysis of lymphocyte-subsets are warranted for dogs with erosive
IMPA.
For
each IMPA-affected dog, information extracted from the medical
record included signalment, diagnostic test results, radiographic
findings, and treatments administered. Dogs were classified as
having erosive IMPA if review of radiographs revealed the presence
of bone lysis in multiple joints, and descriptive data were
generated for those dogs. All available direct smears of synovial
fluid samples underwent cytologic evaluation. The synovial fluid
total nucleated cell count and WBC differential count were estimated
and compared between dogs with erosive IMPA and dogs with nonerosive
IMPA. Results: 13 of 79 (16%) dogs had erosive IMPA. Dogs with
erosive IMPA had a mean ± SD age of 7.1 ± 2.4 years and body weight
of 8.3 ± 3.4 kg (18.3 ± 7.5 lb). All 13 dogs had erosive lesions in
their carpal joints. (See left carpus of an 8-year-old spayed
female Cavalier King Charles Spaniel with subjectively moderate
subchondral bone lysis accompanied by severe synovial swelling -- in
x-ray at right).The estimated median synovial fluid lymphocyte
count for dogs with erosive IMPA was significantly greater than that
for dogs with nonerosive IMPA. All dogs received immunosuppressive
therapy with leflunomide (n = 9), prednisone (3), or
prednisone-azathioprine (1). Conclusions & Clinical Relevance:
Results indicated erosive IMPA most commonly affected the carpal
joints of middle-aged small-breed dogs. Further genetic analyses and
analysis of lymphocyte-subsets are warranted for dogs with erosive
IMPA.
Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Katharine L. Anderson, Dan G. O'Neill, David C. Brodbelt, David B. Church, Richard L. Meeson, David Sargan, Jennifer F. Summers, Helen Zulch, Lisa M. Collins. Sci. Repts. April 2018;8:5641. Quote: Osteoarthritis is the most common joint disease diagnosed in veterinary medicine and poses considerable challenges to canine welfare. This study aimed to investigate prevalence, duration and risk factors of appendicular osteoarthritis in dogs under primary veterinary care in the UK. The VetCompassTM programme collects clinical data on dogs attending UK primary-care veterinary practices. The study included all VetCompassTM dogs under veterinary care during 2013. Candidate osteoarthritis cases were identified using multiple search strategies. A random subset was manually evaluated against a case definition. Of 455,557 study dogs, 16,437 candidate osteoarthritis cases were identified; 6104 (37%) were manually checked and 4196 (69% of sample) were confirmed as cases. Additional data on demography, clinical signs, duration and management were extracted for confirmed cases. Estimated annual period prevalence (accounting for subsampling) of appendicular osteoarthritis was 2.5% (CI95: 2.4-2.5%) equating to around 200,000 UK affected dogs annually. Risk factors associated with osteoarthritis diagnosis included breed (e.g. Labrador, Golden Retriever), being insured, being neutered, of higher bodyweight and being older than eight years. Duration calculation trials suggest osteoarthritis affects 11.4% of affected individuals' lifespan, providing further evidence for substantial impact of osteoarthritis on canine welfare at the individual and population level. ... Prevalence estimate. The estimated annual period prevalence of osteoarthritis diagnosis in dogs under primary veterinary care in the UK during 2013 was 2.5% (CI95: 2.4-2.5%). Prevalence of the most frequently affected breeds was also calculated with the most prevalent breeds being large breeds, specifically: Golden Retriever (7.7% of all Golden retrievers), Labrador Retriever (6.1% of all Labradors), Rottweiler (5.4% of all Rottweilers) and German Shepherd Dog (4.9% of all German Shepherds). ... Cavalier King Charles (2.42%).
Spontaneous Septic Arthritis of Canine Elbows: Twenty-One Cases. Ben Mielke, Eithne Comerford, Kate English, Richard Meeson. Vet Comp Orthop Traumatol. November 2018;31(6):488-493. Quote: Objective: This study provides information on clinical features, diagnosis, treatment and associated risk factors of spontaneous septic elbow arthritis in the dog. Methods: Medical records between March 2007 and June 2015 were searched for cases of spontaneous septic elbow arthritis with a diagnosis based on clinical signs, arthrocentesis, cytological and microbiological analysis of elbow joint synovial fluid, radiography and outcome following treatment. Results: Twenty-one cases of septic arthritis were identified [including one cavalier King Charles spaniel]. Pre-existing osteoarthritis was present in 14/15 elbows for which diagnostic imaging was available. Although all cases had increased neutrophil count on synovial fluid cytology, culture was only positive in 12/21. Despite initial improvement in lameness scores (pre-treatment 9/10 [range: 1-10] versus post-treatment 3/10 [range: 1-5]), 11/12 had residual long-term lameness. Recurrence of infection was noted in 3/12 elbows for which long-term (>8 weeks) follow-up was available. There was an acute mortality rate of 2/21 associated with severe systemic sepsis. Clinical significance: Septic arthritis, even in the absence of pyrexia, should be considered as a major differential diagnosis in middle aged, large breed dogs, with pre-existing elbow arthritis, that suffer an acute onset lameness, with elbow joint effusion and discomfort. Antibiotic therapy alone was effective for treatment with high initial response rates. Chronic lameness post-treatment was common, and a high rate of recurrence was seen with 3/12 dogs suffering more than one episode.
Evaluation of the effects of undenatured type II collagen (UC-II) as compared to robenacoxib on the mobility impairment induced by osteoarthritis in dogs. Stabile M, Samarelli R, Trerotoli P, Fracassi L, Lacitignola L, Crovace A, et al. Vet. Sci. September 2019; doi: 10.3390/vetsci6030072. Quote: Osteoarthritis (OA) is a chronic disease that requires a multimodal therapeutic approach. The aim of this study was to evaluate the effects of undenatured type II collagen (UC-II) as compared to robenacoxib in dogs affected by OA. Our hypothesis was that the two compounds would be similar (non-inferiority) in improving mobility. To test this hypothesis, a complete orthopedic examination, x-ray and the Liverpool Osteoarthritis in Dogs (LOAD) survey were performed in dogs affected by OA before and after the treatments. The study was designed as a clinical, randomized, controlled and prospective study. Sixty client-owned dogs were randomized in the R group (n = 30, robenacoxib 1 mg/kg/day for 30 days) and in the UC-II group (n = 30, UC-II 1 tablet/day for 30 days). Thirty days after the beginning of the treatment (T30), the dogs were reassessed for the LOAD, MOBILITY and CLINICAL scores. Based on the data obtained from the study, a significant reduction in LOAD and MOBILITY scores was recorded between T0 and T30 with a similar magnitude among the two groups (R = 31.5%, p < 0.001; UC-II = 32.7%, p = 0.013). The results of this study showed that UC-II and robenacoxib were able to similarly improve mobility of dogs affected by OA.
The Use of Cannabidiol-Rich Hemp Oil Extract to Treat Canine Osteoarthritis-Related Pain: A Pilot Study. Lori Kogan, Peter Hellyer, Robin Downing. AHVMA J. March 2020;58(Spring):42-45. Quote: The objective of this 90-day pilot clinical trial was to assess the impact of a full-spectrum product containing hemp extract and hemp seed oil on dogs with chronic mal adaptive pain. A total of 37 dogs diagnosed with chronic maladaptive pain primarily as a result of osteoarthritis were enrolled in the study. The dogs were given an initial physical examination that included systematic pain palpation, mapping of pain patterns, informal gait analysis, metabolic profile, and owner interview. The same palpa tions and mappings were performed during each biweekly assessment to identify trends, chart progress, and inform dose adjustments. ... Among these 30 dogs, the dose of CBD needed to achieve a positive effect ranged from 0.3 up to 4.12 mg/kg BID. The 2 dogs in the study requiring the highest dose of the CBD product were both Cavalier King Charles spaniels (not related to one another), and neither of these dogs experienced any changes/elevations in liver enzymes. It is unclear why some patients responded to a very small dose of the CBD product (0.3 mg/kg per dose), whereas the majority required dosing in the range of 1 to 2 mg/kg per dose. ... The metabolic parameters were repeated at the end of the study. Of the 32 dogs that completed the study, 30 dogs demonstrated improved pain support. Of the 23 dogs in the study that were taking gabapentin at the time of enrollment, 10 dogs were able to discontinue the gabapentin, and an additional 11 dogs were able to have their daily dose reduced with the addition of the cannabidiol (CBD) oil. Conclusion: The addition of a hemp- derived CBD oil appears to positively affect dogs with chronic maladaptive pain by decreasing their pain, thereby improving their mobility and quality of life. The reduction in gabapentin dose may be the result of changes in analgesia and/or sedation with the addition of the hemp oil extract.
Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis. Federica Alessandra Brioschi, Federica Di Cesare, Daniela Gioeni, Vanessa Rabbogliatti, Francesco Ferrari, Elisa Silvia D'Urso, Martina Amari, Giuliano Ravasio. Animals. August 2020; doi: 10.3390/ani10091505. Quote: The aim of this study was to evaluate the efficacy of oral transmucosal (OTM) cannabidiol (CBD), in addition to a multimodal pharmacological treatment for chronic osteoarthritis-related pain in dogs. Twenty-one dogs were randomly divided into two groups: in group CBD (n = 9), OTM CBD (2 mg kg−1 every 12 h) was included in the therapeutic protocol (anti-inflammatory drug, gabapentin, amitriptyline), while in group C (n = 12), CBD was not administered. Dogs were evaluated by owners based on the Canine Brief Pain Inventory scoring system before treatment initiation (T0), and one (T1), two (T2), four (T3) and twelve (T4) weeks thereafter. Pain Severity Score was significantly lower in CBD than in C group at T1 (p = 0.0002), T2 (p = 0.0043) and T3 (p = 0.016). Pain Interference Score was significantly lower in CBD than in C group at T1 (p = 0.0002), T2 (p = 0.0007) and T4 (p = 0.004). Quality of Life Index was significantly higher in CBD group at T1 (p = 0.003). The addition of OTM CBD showed promising results. Further pharmacokinetics and long-term studies in larger populations are needed to encourage its inclusion into a multimodal pharmacological approach for canine osteoarthritis-related pain.
A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Chris D. Verrico, Shonda Wesson, Vanaja Konduri, Colby J. Hofferek, Jonathan Vazquez-Perez, Emek Blair, Kenneth Dunner Jr, Pedram Salimpour, William K. Decker, Matthew M. Halpert. Pain. September 2020; doi: 10.1097/j.pain.0000000000001896. Quote: Over the last 2 decades, affirmative diagnoses of osteoarthritis (OA) in the United States have tripled due to increasing rates of obesity and an aging population. Hemp-derived cannabidiol (CBD) is the major nontetrahydrocannabinol component of cannabis and has been promoted as a potential treatment for a wide variety of disparate inflammatory conditions. Here, we evaluated CBD for its ability to modulate the production of proinflammatory cytokines in vitro and in murine models of induced inflammation and further validated the ability of a liposomal formulation to increase bioavailability in mice and in humans. Subsequently, the therapeutic potential of both naked and liposomally encapsulated CBD was explored in a 4-week, randomized placebo-controlled, double-blinded study in a spontaneous canine model of OA. In vitro and in mouse models, CBD significantly attenuated the production of proinflammatory cytokines IL-6 and TNF-a while elevating levels of anti-inflammatory IL-10. In the veterinary study, CBD significantly decreased pain and increased mobility in a dose-dependent fashion among animals with an affirmative diagnosis of OA. Liposomal CBD (20 mg/day) was as effective as the highest dose of nonliposomal CBD (50 mg/day) in improving clinical outcomes. Hematocrit, comprehensive metabolic profile, and clinical chemistry indicated no significant detrimental impact of CBD administration over the 4-week analysis period. This study supports the safety and therapeutic potential of hemp-derived CBD for relieving arthritic pain and suggests follow-up investigations in humans are warranted.
Undenatured type II collagen mitigates inflammation and cartilage degeneration in healthy Labrador retrievers during an exercise regimen. Varney JL, Fowler JW, Coon CN. Transl. Anim. Sci. May 2021; doi: 10.1093/tas/txab084. Quote: The aim of this experiment was to evaluate the effect of undenatured type II collagen supplementation on inflammation and cartilage degeneration after exercise in healthy dogs. Forty healthy Labrador Retrievers (20 male/20 female; range 5-12 yr; average 8 yr) were sorted into two groups: undenatured type II collagen group receiving 40 mg UC-II (10 mg collagen type II/min. 3% undenatured type II collagen; Lonza Consumer Health, Inc.) and placebo group receiving 40 mg maltodextrin daily by capsule. After 2-wk loading, all dogs began an 11-wk endurance exercise regimen consisting of two weekly runs, starting at 5 km and increasing incrementally to 8 km, with one final 16 km run. Blood samples were collected at baseline, pre and post first 5 km run, and pre- and post-16 km run. Activity per kilometer was greater in male undenatured type II collagen vs. male placebo over all runs (P = 0.004), and average moving speed was greater in all undenatured type II collagen dogs compared with placebo over all runs (P < 0.001). Hematology analysis indicated that during the first insult, undenatured type II collagen dogs had a greater lymphocyte count (P < 0.001) and lymphocyte percentage (P = 0.001) vs. placebo dogs. Undenatured type II collagen dogs had a lesser neutrophil percentage (P = 0.042) and neutrophil to lymphocyte ratios (P = 0.001) compared to placebo dogs. For the final insult, undenatured type II collagen dogs had greater lymphocyte percentage (P = 0.013) and lesser mean corpuscular hemoglobin concentration (P = 0.043) compared with placebo dogs. Both groups had significant changes between timepoints for several hematological parameters. Biomarker IL-6 was lesser in undenatured type II collagen dogs compared with placebo at post 5 km (P = 0.037). Cartilage oligomeric matrix protein (COMP) was lesser in undenatured type II collagen dogs at post 16 km (P = 0.023), and only the placebo dogs had a significant increase in COMP from pre to post 16 km (P = 0.021). In summary, Labrador Retrievers supplemented with undenatured type II collagen had decreased inflammation and cartilage degeneration compared with nonsupplemented dogs during exercise.
A multiple-session mesotherapy protocol for the management of hip osteoarthritis in police working dogs. Joao C. Alves, Ana Santos, MSc, Patrícia Jorge, P. Lafuente. Amer. J. Vet. Res. January 2023; doi: 10.2460/ajvr.22.08.0132. Quote: Objective: To describe the effect of a mesotherapy protocol in dogs with osteoarthritis. Animals: 30 dogs. Procedures: Dogs were randomly assigned to a control (CG; n = 10) or a mesotherapy group (MG; 20). CG received meloxicam for 70 days. MG was treated with a combination of lidocaine, piroxicam, and thiocolchicoside, injected in intradermal points. Seven treatment sessions were conducted. Response to treatment was measured with different instruments: the Canine Brief Pain Inventory (divided into Pain Interference Score [PIS] and Pain Severity Score [PSS]), Liverpool Osteoarthritis in Dogs (LOAD), and Canine Orthopedic Index (COI; divided into function, gait, stiffness, and quality of life), at time 0 (T0), +15 days, +30 days, +60 days, and +90 days after T0. At each time point, the results of the 2 groups with each instrument were analyzed with the Wilcoxon signed ranks test, P < .05. Kaplan-Meier estimators were compared with the Breslow test. Results: Dogs had a mean age of 6.9 ± 2.7 years and a body weight of 31.0 ± 6.4 kg. Hip osteoarthritis was classified as mild (n = 9), moderate (17), or severe (4). No differences were found at T0. Better results were observed in MG at +15 days (P < .01 for PSS and PIS, P = .03 for function), +30 days (P = .01 for PIS and LOAD, P = .03 for PSS, and P = .04 for function, gait, and COI), +60 days (P < .01 for PSS and PIS, P = .01 for LOAD, and P = .02 for function), and +90 days (P = .01 for PSS and PIS, P = .03 for LOAD, and P = .04 for function). Kaplan-Meier estimators showed MG had longer periods with better results than CG in various scores. Clinical Relevance: This mesotherapy protocol reduced pain scores and other clinical metrology instrument scores lasting for longer periods.
The effect of oral administration of undenatured type II collagen on
monosodium iodoacetate-induced osteoarthritis in young and old rats.
Sahin E, Orhan C, Erten F, Saiyed Z, Azari EK, Durkee S, et al. Sci.
Rep. April 2023; doi:
10.1038/s41598-023-33763-2. Quote: We
investigated whether different doses of undenatured type II collagen
(undenatured collagen, UC-II) help improve monosodium iodoacetate
(MIA)-induced (osteoarthritis) OA in young and old rats. A total of
70 rats were divided into five groups: (1) control; (2) MIA (a
single intra-articular injection of MIA); (3)-(5) MIA+ Undenatured
Collagen with various oral doses (0.66, 1.33, and 2 mg/kg). The
results showed that all doses of undenatured collagen in both age
groups reduced knee diameter, while the two higher doses (1.33 mg/kg
and 2 mg/kg) reduced the Mankin score and increased most gait
measurements as early as day 14 compared to the MIA rats. However,
the 2 mg/kg dose showed the best efficacy in improving Mankin score
and gait measurements by 28 days post-OA induction. In young but not
old rats, all doses of undenatured collagen reduced the
Kellgren-Lawrence score compared to the MIA group. Undenatured
collagen reduced the levels of most inflammatory and cartilage
breakdown markers in serum and knee joint cartilage in both age
groups. In conclusion, this data suggests that while all doses of
undenatured collagen supplementation may ameliorate MIA-induced OA
symptoms, the higher doses showed faster improvement in gait
measurements and were more efficacious for overall joint health in
rats.
Efficacy and safety of cannabidiol for the treatment of canine osteoarthritis: a systematic review and meta-analysis of animal intervention studies. Chanthawat Patikorn, Osot Nerapusee, Kumpanart Soontornvipart, Kanta Lawonyawut, Kachapong Musikpodok, Kanisorn Waleethanaphan, Puree Anantachoti. Front. Vet. Sci. September 2023; doi: 10.3389/fvets.2023.1248417. Quote: Introduction: Canine osteoarthritis (OA) is a degenerative disease with chronic inflammation of internal and external joint structures in dogs. Cannabis spp. contains cannabidiol (CBD), a substance known for various potential indications, such as pain relief and anti-inflammatory in various types of animals, including dogs with OA. As CBD is increasingly in the spotlight for medical use, we aimed to perform a systematic review and meta-analysis to evaluate the efficacy and safety of CBD in treating canine OA. Methods: We searched PubMed, Embase, Scopus, and CAB Direct for animal intervention studies investigating the effects of CBD for canine OA from database inception until February 28, 2023. Study characteristics and findings were summarized. A risk of bias in the included studies was assessed. Meta-analyses were performed using a random-effects model to estimate the effects of CBD on pain scores (0-10), expressed as mean difference (MD) and 95% confidence interval (95% CI). Certainty of evidence was assessed using GRADE. Results: Five articles were included, which investigated the effects of CBD in 117 dogs with OA. All studies were rated as having a high risk of bias. CBD products varied substantially, i.e., oral full-spectrum CBD oil in four studies, and isolated CBD oil and liposomal CBD oil in another study. Treatment duration varied from 4-12 weeks. Meta-analyses of three studies found that, in dogs with OA, treatment with oral full-spectrum CBD oil may reduce pain severity scores (MD; −0.60, 95% CI; −1.51 to 0.31, I2 = 45.64%, p = 0.19) and pain interference scores (MD; −1.52, 95% CI; −3.84 to 0.80, I2 = 89.59%, p = 0.20) but the certainty of evidence was very low. CBD is generally considered safe and well-tolerated in the short-run, with few mild adverse events observed, such as vomiting and asymptomatic increase in alkaline phosphatase level. Conclusion: CBD is considered safe for treating canine OA. CBD may reduce pain scores, but the evidence is very uncertain to conclude its clinical efficacy. High-quality clinical trials are needed to further evaluate the roles of CBD in canine OA.
Librela (Beransa) - Wonder Drug Or Disaster In The Making? Edward Bassingthwaighte. Dogs Naturally. October 2023. Quote: In my professional opinion, there is no way that Librela should ever be considered as a first treatment or intervention for arthritis in dogs.
A blinded, randomized and controlled multicenter field study investigating the safety and efficacy of long-term use of enflicoxib in the treatment of naturally occurring osteoarthritis in client-owned dogs. Josep Homedes, Marion Ocak, Sebastian Riedle, Marta Salichs. Frontiers in Vet. Sci. February 2024; doi: 10.3389/fvets.2024.1349901. Quote: Background Enflicoxib is a COX-2 selective NSAID shown to be efficacious and safe in the treatment of pain and inflammation associated with canine osteoarthritis (OA) in clinical studies of 6 weeks duration. Objective This prospective, multisite, blinded, randomized, placebo-controlled, parallel-group field study aimed to confirm the safety and efficacy of enflicoxib in long-term canine OA treatments. Animals A total of 109 client owned dogs with clinical and radiographic signs of OA for at least 3 weeks were enrolled with 78 dogs completing all study visits. Methods Dogs were randomized at a 3:1 ratio to receive enflicoxib (n = 83) or placebo (n = 26) once weekly during 6 months. Dogs underwent veterinary assessments from Day 0 to Day 189 using a clinical sum score (CSS). Efficacy was also assessed by the owners using the Canine Brief Pain Inventory (CBPI). Safety was assessed clinically and by repeated blood and urine sample analysis. The efficacy outcome measure was the treatment response according to the CSS and secondarily the treatment response according to the CBPI. The primary safety outcome was the incidence of adverse events (AEs) and secondarily the evolution of the clinical pathology parameters. Results Percentages of CSS responders for enflicoxib were 71.6; 74.6 and 71.6% on Days 44, 135 and 189 respectively, always showing statistically significant differences (p < 0.05) vs. placebo (41.7, 33.3, and 20.8% respectively). Treatment response according to owner assessments followed the same pattern, achieving significant differences compared to placebo after 2 weeks of treatment. The incidence and type of AEs were as described in previous enflicoxib studies of shorter duration and as for other NSAIDs, with no tendency to increase over time. No relevant changes in hematology, biochemistry or urine parameters were observed. Conclusions and clinical relevance Enflicoxib safety and efficacy profile is maintained after a long-term treatment, which together with its weekly administration, makes it a good alternative for the chronic treatment of dogs with naturally occurring OA.
Cannabidiol plus krill oil supplementation improves chronic stifle osteoarthritis in dogs: A double-blind randomized controlled trial. K. Soontornvipart, P. Wongsirichatchai, A. Pongphuwanun, K. Chatdarong, S. Vimolmangkang. Vet. J. August 2024; doi: 10.1016/j.tvjl.2024.106227. Quote: Osteoarthritis (OA) is the most common orthopedic disorder characterized by chronic inflammation and pain in dogs and cats. Cannabis spp. contains cannabidiol (CBD), a substance with pain relief and anti-inflammatory properties in different animals including dogs with OA. The use of CBD supplements has been increasingly intertwining in veterinary medicine. This study aimed to evaluate the clinical efficacy of CBD + krill oil-supplemented biscuit against canine OA. In total, 30 dogs with stifle OA were randomized and divided into the placebo, krill oil, and CBD + krill oil groups. The Canine Brief Pain Inventory questionnaire was used to evaluate the efficacy of each treatment against pain. Stifle temperature was monitored to identify degrees of stifle inflammation. Two and one dogs in the placebo group were excluded from the study due to worsening lameness and increased pain interference score (PIS) and pain severity score (PSS) at days 14 and 28, respectively. The PIS and PSS scores of the krill oil and CBD + krill oil groups gradually and significantly improved after two weeks of treatment. The CBD + krill oil group had better PIS and PSS scores than the placebo and krill oil groups. However, there was no statistically significant difference in the PIS and PSS scores between the krill oil and CBD + krill oil groups. The stifle temperature of the three groups at different periods did not significantly differ. In conclusion, CBD + krill oil supplements are safe against canine OA. CBD can reduce pain and inflammation. Highlights: • CBD + krill oil-supplemented biscuit significantly reduced pain and inflammation in OA dogs. • Krill oil or CBD + krill oil biscuits both reduced pain, with the latter showing slightly better results. • No side effects were observed when giving the dog the CBD + krill oil-supplemented biscuit.
A proposed framework for practical multimodal management of osteoarthritis in growing dogs. Denis J. Marcellin-Little, Donald A. Hulse, Janice L. Huntingford, Tamara Grubb, Matthew W. Brunke, Arielle Pechette Markley, Bethany Frank. Front. Vet. Sci. April 2025; doi: 10.3389/fvets.2025.1565922. Quote: Osteoarthritis (OA) is a ubiquitous problem affecting dog joints, particularly the hip, elbow, stifle, and spine. OA most often results from developmental orthopedic problems such as hip dysplasia, elbow dysplasia, and patellar luxation and from injuries to the cranial cruciate ligament. Several management approaches have been proposed to manage OA, including steps to modulate growth, physical activity, and exercise, nutrition and nutritional supplementation, medications, physical rehabilitation, and surgical procedures. This article is the first in a series of articles that propose steps for practical OA management in dogs at various life stages. The review presented here focuses on growing dogs. The text describes the early pathophysiology and diagnosis of OA. The physical, nutritional, analgesic, and surgical management options of OA in growing dogs are presented. The application of these management options is described for three dogs. The overall approach to the management of OA in growing dogs is discussed.
Musculoskeletal adverse events in dogs receiving bedinvetmab (Librela). Mike Farrell, Felix W. A. Waibel, Ines Carrera, Giliola Spattini, Louise Clark, Robert J. Adams, Dirsko J. F. Von Pfeil, Ricardo J. R. De Sousa, Diego Bobis Villagrà, Maria Amengual-Vila, Annalisa Paviotti, Rob Quinn, Justin Harper, Stephen P. Clarke, Christopher J. Jordan, Michael Hamilton, Andy P. Moores, Mark Irwin Greene. Front. Vet. Sci. May 2025; doi: 10.3389/fvets.2025.1581490. Quote: Objectives: To conduct a specialist-led disproportionality analysis of musculoskeletal adverse event reports (MSAERs) in dogs treated with bedinvetmab (Librela™) compared to six comparator drugs with the same indication. Furthermore, to report the findings from a subset of dogs whose adverse event (AE) data underwent independent adjudication by an expert panel. Study design: Case-control study and case series analysis. Sample population: The European Medicines Agency's EudraVigilance database (2004-2024) and 19 client-owned dogs. Methods: An EBVS® Veterinary Specialist in Surgery individually reviewed all MSAERs to Librela™, Rimadyl®, Metacam®, Previcox®, Onsior®, Galliprant®, and Daxocox® (2004-2024). The primary null hypothesis was that Librela's MSAER rate would not exceed that of comparator drugs by more than 50%. The secondary hypothesis was that MSAER would surge and taper following the launch of new drugs. Results: The disproportionality analysis did not support the hypotheses. Ligament/tendon injury, polyarthritis, fracture, musculoskeletal neoplasia, and septic arthritis were reported ~9-times more frequently in Librela-treated dogs than the combined total of dogs treated with the comparator drugs. A review of 19 suspected musculoskeletal adverse events (MSAEs) by an 18-member expert panel unanimously concluded a strong suspicion of a causal association between bedinvetmab and accelerated joint destruction. Conclusion: This study supports recent FDA analyses by demonstrating an increased reporting rate of musculoskeletal adverse events in dogs treated with Librela. Further investigation and close clinical monitoring of treated dogs are warranted. Impact: Our findings should serve as a catalyst for large-scale investigations into bedinvetmab's risks and pharmacovigilance.
Commentary: Musculoskeletal adverse events in dogs receiving bedinvetmab (Librela). Matthew Warren Brunke, Curtis Wells Dewey. Front. Vet. Sci. July 2025; doi: 10.3389/fvets.2025.1628681.
Rapidly progressive osteoarthritis (RPOA) in companion animals treated with bedinvetmab (Librela™): an expected pathophysiological phenomenon or a cause for concern? Ali Mobasheri, Peter Hanson, Jonathan Larkin. Front. Vet. Sci. August 2025; doi: 10.3389/fvets.2025.1640217. Quote: The advent of anti-nerve growth factor (NGF) monoclonal antibodies, such as bedinvetmab (Librela™), has marked a significant advancement in the management of osteoarthritis (OA) pain in dogs. First approved for use in October 2020 (EU) and May 2023 (U.S.), bedinvetmab offers a novel mechanism of action by targeting NGF, a key mediator in pain pathways, thereby providing relief for canine OA patients.While clinical trials have demonstrated the efficacy and safety of bedinvetmab, post-marketing surveillance has brought to light reports of adverse events, including neurological signs such as ataxia, seizures, and paresis, as well as musculoskeletal issues like lameness. Similar observations have been made in humans with OA treated with NGF neutralizing antibodies such as Tanezumab, which was originally developed for the treatment of human OA (6). These observations have collectively raised concerns about the potential for rapidly progressive OA (RPOA) in dogs undergoing treatment with bedinvetmab and humans receiving Tanezumab.The term RPOA has recently become a focal point in OA drug development, particularly following its association with the anti-NGF class of analgesics. RPOA is characterized by an accelerated deterioration of joint structures, leading to severe pain and functional impairment. In human medicine, RPOA has been associated with anti-NGF therapies, particularly when used concomitantly with nonsteroidal anti-inflammatory drugs (NSAIDs). The pathophysiology is thought to involve the suppression of pain signals, resulting in increased joint usage and subsequent structural damage. In veterinary medicine, the occurrence of RPOA in dogs treated with bedinvetmab remains a subject of investigation, monitoring and scrutiny. Recent case reports have reported rapid joint deterioration in dogs with elbow OA, following the initiation of bedinvetmab therapy. While case reports do not establish causality, they underscore the need for vigilance and further safety monitoring. The potential for RPOA in canine patients necessitates a cautious approach, especially regarding the concurrent use of NSAIDs, despite previous reports of acceptable safety. Although the safety of combining bedinvetmab with NSAIDs has not been fully established, some practitioners have employed short-term NSAID therapy during the initiation of bedinvetmab treatment. Given the experiences in human medicine, it may be prudent to limit or closely monitor such combinations until more definitive data are available.The identification of RPOA in dogs is unfortunately complicated by the lack of standardized diagnostic criteria and the variability in clinical presentations. Advanced imaging modalities and longitudinal structural studies are essential to differentiate RPOA from the natural progression of OA and to elucidate any potential links to anti-NGF therapies. This reminds us of the crucially important work that was carried out by the Nobel Prize winner Dr Rita Levi-Montalcini 1 and her colleagues almost 30 years ago, following the discovery of NGF, warning that NGF must be viewed as a multifactorial mediator that modulates more than pain responses, and is involved in neuroimmune-endocrine functions of vital importance to the regulation of physiological homeostatis, with potential involvement in pathological processes deriving from dysregulation of either local or systemic homeostatic balances (12). Any treatment that blocks the production and the signal of this vital growth factor requires long-term safety monitoring in humans and in companion animals.In conclusion, while bedinvetmab represents a promising option for managing OA pain in dogs, the emergence of adverse events resembling RPOA warrants careful consideration. Veterinarians should remain alert to signs of rapid joint deterioration in companion animals receiving bedinvetmab and they must report such cases to appropriate pharmacovigilance systems. Case reports are generally regarded as week in terms of scientific and medical evidence and we need better processes for integrating them into scientific and medical knowledge. The number of adverse cases associated with bedinvetmab use are generally low, but they do occur. Therefore further research is imperative to determine the incidence, risk factors, and pathogenesis of RPOA in canine patients, thereby ensuring the safe and effective use of anti-NGF therapies in veterinary practice.


CONNECT WITH US